Abstract
Objectives
MDMA users have impaired verbal memory, and voxel-based morphometry has demonstrated decreased gray matter in Brodmann area (BA) 18, 21 and 45. Because these regions play a role in verbal memory, we hypothesized that MDMA users would show altered brain activation in these areas during performance of an fMRI task that probed semantic verbal memory.
Methods
Polysubstance users enriched for MDMA exposure participated in a semantic memory encoding and recognition fMRI task that activated left BA 9, 18, 21/22 and 45. Primary outcomes were percent BOLD signal change in left BA 9, 18, 21/22 and 45, accuracy and response time.
Results
During semantic recognition, lifetime MDMA use was associated with decreased activation in left BA 9, 18 and 21/22 but not 45. This was partly influenced by contributions from cannabis and cocaine use. MDMA exposure was not associated with accuracy or response time during the semantic recognition task.
Conclusions
During semantic recognition, MDMA exposure is associated with reduced regional brain activation in regions mediating verbal memory. These findings partially overlap with prior structural evidence for reduced gray matter in MDMA users and may, in part, explain the consistent verbal memory impairments observed in other studies of MDMA users.
Keywords: Ecstasy (MDMA), drug abuse, semantic memory, verbal memory, fMRI, neuroimaging
INTRODUCTION
After a period of decline, use of the recreational “club drug” 3, 4-methylenedioxymethamphetamine (MDMA or “Ecstasy”) has been rising in the United States since 2005 and recent data suggests that over 12 million Americans have used MDMA (Johnston et al. 2008;Substance Abuse and Mental Health Services Administration 2003). Ecstasy remains highly popular throughout the world, especially in North America, Western Europe and Oceania (United Nations Office on Drugs and Crimes 2008).
Numerous animal studies support MDMA-induced long term serotonergic (5-HT) alterations through degeneration of presynaptic axon terminals and depletion of brain 5-HT upon exposure to MDMA of adequate magnitude and chronicity (Gibb et al. 1990;Green et al. 2003;Ricaurte et al. 2000). This has led to significant concerns regarding the neurotoxic potential of MDMA. However, loss of brain 5-HT markers can occur in the absence of axonal loss (Fantegrossi et al. 2004;Wang et al. 2004;Wang et al. 2005), suggesting that MDMA may produce functional consequences for 5-HT neurotransmission even in the absence of axon degeneration. Animal data have also shown an association between MDMA use and long term behavior change, which may be mediated by chronic alterations in the 5-HT system (Easton et al. 2006). These data raise significant concern about the possibility of chronic neurological effects of MDMA use in humans.
There is considerable evidence that MDMA induces alterations in the 5-HT system in humans, including reduced binding to the serotonin reuptake transporter (5-HTT) (de Win et al. 2004;McCann et al. 1998;McCann et al. 2005;McCann et al. 2008;Reneman et al. 2001a;Semple et al. 1999), reduced levels of the 5-HT breakdown product 5-hydroxyindoleacetic acid (5-HIAA) (McCann et al. 2000) and upregulation of the 5-HT2A receptor (Reneman et al. 2000b;Reneman et al. 2000a;Reneman et al. 2002). Evidence from human studies suggests that altered serotonergic neurotransmission is long-lasting (Curran et al. 2003;Reneman et al. 2002), although some studies suggest that partial improvement may occur with long-term abstinence (Semple et al. 1999;Thomasius et al. 2003). Because the chronicity of the alterations induced by MDMA remain unclear, and the partial improvements observed with abstinence could reflect pre-existing differences, the issue of potential neurotoxicity in humans remains controversial.
Many, but not all (Back-Madruga et al. 2003;Gouzoulis-Mayfrank et al. 2005), studies have found evidence of impairment across multiple neurocognitive domains including verbal working memory (Jacobsen et al. 2004), episodic memory (Morgan 2000) and visual memory (Back-Madruga et al. 2003) in subjects with a history of MDMA exposure. Recent meta-analyses found effect sizes ranging from small to substantial for impaired verbal memory and for short and long term memory impairment in MDMA users compared to non-MDMA using control subjects (Kalechstein et al. 2007;Laws et al. 2007). A recent prospective cohort study compared subjects who started using MDMA with non-MDMA polysubstance users, and found significantly reduced immediate and delayed verbal recognition on the Ray Auditory-Verbal Learning Test (RAVLT) in the MDMA users (Schilt et al. 2007).
Several associative studies have shown a relationship between neurocognitive deficits in MDMA users and underlying neural alterations. Bolla et al. studied subjects abstinent from MDMA for a median of 4 weeks and found that verbal and visual memory deficits correlated with a reduction in levels of the 5-HT metabolite 5-HIAA (Bolla et al. 1998). Using magnetic resonance spectroscopy (MRS), Reneman et al. found significant deficits in delayed word recall in MDMA users compared to control subjects that were strongly associated with a reduced NAA (N-acetylaspartate)/Cr (creatine) ratio in the prefrontal cortex (Reneman et al. 2001d). McCann et al. (McCann et al. 1998) found that the relationship between 5-HTT binding and cognition seen in non-MDMA users was disrupted in MDMA users, suggesting that MDMA disrupts the relationship between 5-HT function and cognition. However a causal link between neural alterations and neurocognitive dysfunction remains to be established.
Imaging studies have also supported the association between MDMA exposure and functional brain alterations. Reneman et al. found increased regional cortical blood flow and increased apparent diffusion coefficient of water in the globus pallidi of MDMA users who had been abstinent for at least 3 weeks (Reneman et al. 2001c). This is consistent with subchronic vasodilation in recently abstinent MDMA users. Using FDG PET, Obrocki et al. found significantly reduced left hippocampal metabolism in MDMA users compared to oncology controls (Obrocki et al. 1999). 2 subsequent reports using similar techniques found reduced resting metabolism in bilateral caudate/putamen and in the left amygdala in MDMA users compared to oncology controls (Buchert et al. 2001;Obrocki et al. 2002). Using an N-back fMRI paradigm activating working memory, Daumann et al. found significantly greater activation in the right superior parietal lobe and significantly reduced activation in left posterior cingulate cortex, bilateral inferior temporal gyri and bilateral angular gyri in recently abstinent relatively pure MDMA users compared to controls (Daumann et al. 2003). Using a prospective technique in a similar study, Daumann et al. found increased activation in parietal cortex compared to baseline assessment activation in subjects who continued to use MDMA or amphetamines. The activation was positively correlated with lifetime MDMA use (Daumann, Jr. et al. 2004). These studies support reduced resting brain metabolism in selected brain regions and alterations in regional brain activation that are associated with MDMA exposure.
Cowan et al. (Cowan et al. 2003) used voxel-based morphometry (VBM) to study neocortical gray matter volume in MDMA polysubstance users and non-MDMA polysubstance users and found decreased gray matter concentrations in bilateral Brodmann area (BA) 18, left BA 21 and left BA 45. Interestingly, a recent positron emission tomography (PET) study (Lee et al. 2002) demonstrated that similar regions (BA 9, 18, 21/22 and 45) were involved in semantic memory. Based on the findings of gray matter loss in the Cowan et al. report, we hypothesized that BA 18, 21/22 (we included BA 22 because our fMRI task confluently activated both BA 21/22) and 45 would show altered activation during performance of a verbal memory task. We adapted the semantic memory task described by Lee et al. (Lee et al. 2002) with two goals in mind: 1) to produce activation in BA 9, 18, 21/22 and 45 to specifically probe the function of these brain regions and 2) to probe aspects of verbal memory that are potentially impaired in MDMA users. Our paradigm consisted of an encoding phase followed by a recognition phase after a very brief delay; as such, this paradigm tapped elements of semantic and working memory.
Our primary hypothesis was that increased lifetime episodes of MDMA use would be associated with altered percent BOLD signal change in BA 18, 21/22 and 45 but not BA 9 during semantic recognition. Our secondary hypothesis was that increased lifetime episodes of MDMA use would be associated with poorer performance response times and increased errors on the semantic recognition task.
METHODS
To enhance replicability and cross-study comparisons, data are presented (where applicable) as outlined by Poldrack et al. (2008) (Poldrack 2008) in their guidelines for presenting an fMRI study.
Human subjects
We recruited 18 subjects for this study as part of a larger study of neuroimaging in MDMA use via advertisements requesting volunteers aged 18-35 with a history of MDMA or other drug use for an MRI study. Two of the 18 subjects were excluded for excessive motion and technical inadequacy of the volumetric scan. Consequently 16 (6 female; 10 male) right-handed polysubstance users (12 with a history of MDMA use) age 23.6 ± 2.7 years old completed the study and all data reported are from those subjects. We enrolled polydrug users (versus enrolling specific MDMA/non-MDMA users) to conduct within-group exposure/outcome assays of drug exposure. We used Dutch words for the pseudo-word condition (see below) because Dutch words are easily pronounceable to English speakers but do not have semantic meaning. Therefore, exclusion criteria included an understanding of Dutch, lifetime use of between 1 and 5 tablets of MDMA (to exclude subjects with very low-level MDMA use), history of current or past substance or alcohol dependence, history of current or past DSM-IV Axis 1 psychiatric disorder (except substance-induced mood disorder or substance abuse), taking non-illicit psychoactive medications (whether prescription or over-the-counter) within six weeks prior to the study, endocrine abnormalities, history of loss of consciousness for over 30 minutes, contraindications to MR scanning, and a positive urine drug screen on the scan day. Because we were interested in chronic effects of MDMA exposure, all subjects were abstinent from MDMA for at least 2 weeks prior to the fMRI study day.
Ethics approval
The study protocol was approved by the Vanderbilt University Institutional Review Board and conformed to the World Medical Association’s Declaration of Helsinki.
Experimental Design
Task Specification
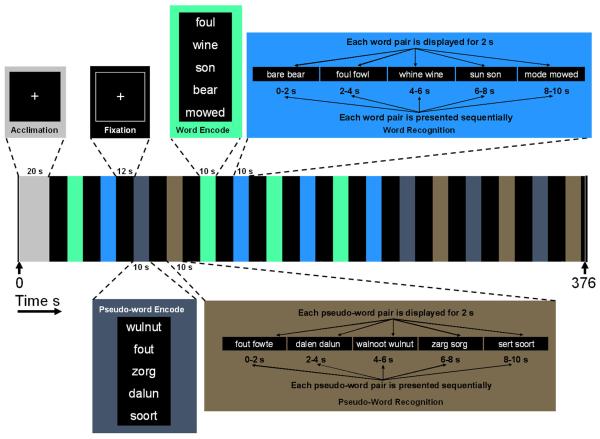
The study was a human within-subjects cross-sectional design assessing the association between self-reports of prior polydrug exposure and outcome variables for fMRI and behavioral response. To probe regional brain activation and behavioral response during semantic encoding we modified a semantic encoding and recognition task developed for PET (Lee et al. 2002). Because our primary goal was to measure regional brain activation, we employed a block stimulus design. The fMRI task consisted of a word and pseudo-word encoding (learning) period and a word and pseudo-word recognition period. We used a block study design consisting of 12 second rest (fixation) periods (Figure 1) interspersed with 10 second blocks of word/pseudo-word encoding or word/pseudo-word recognition. During each encoding block, subjects were instructed to memorize a group of 5 English words or a group of 5 pronounceable pseudo-words in a 10 second encoding period. After a subsequent 12 second fixation period, subjects were presented with homophone pairs (e.g. pray versus prey) in which one of the homophones was novel (not presented during the preceding encoding block) during a 10 second recognition block and asked to correctly identify the word/pseudo-word they had learned during the encoding period. (Dutch words were used as pronounceable pseudo-words and homophones corresponding to Dutch words were synthesized based on English pronunciation). As such, the overall task involved visual and verbal memory encoding and recognition, working memory, and an element of decision-making and motor activation during the forced choice recognition task. Because all visual and motor components of encoding and recognition of words versus pseudo-words were identical except for the fact that the English words have meaning to the subjects, a contrast of regional brain activation during word encoding or recognition versus pseudo-word encoding or recognition was expected to isolate semantic memory.
Figure 1. Semantic Task.
Subjects encoded a block of 5 words or pseudo-words followed by a recall task using novel homophones as distracter images. Subjects were instructed to press a button corresponding to right or left screen location of previously encoded items. Semantic encode: Activation during semantic encoding was defined as (word encode minus pseudo-word encode). Semantic recognition: Activation during semantic recognition was defined as (word recognition minus pseudo-word recognition).
Planned Comparisons
Planned comparisons were the association of percent BOLD signal change for semantic memory in each brain region (left BA 9, 18, 21/22 and 45) with prior drug use. Additional comparisons examined the association of drug use with recognition task variables of reaction time and accuracy.
ROI Analysis Outcome variables
The primary outcome variables were percent BOLD signal change in the a priori chosen brain regions of left BA 9, 18, 21/22 and 45. BA 18, 21/22 and 45 were chosen because of their role in semantic processing and because the earlier VBM study (Cowan et al. 2003) found reduced brain gray matter concentration in these regions (because VBM is a threshold based-assay, it is possible that BA 22 was also affected in our original VBM study. Because our present task evoked a confluent region of activation overlapping BA 21 and 22, we combined these regions in the analysis). BA 9 was included as a “control” region because of its role in semantic processing and because the earlier VBM study did not find an association between MDMA use and BA 9 brain gray matter concentration. Percent BOLD signal change for each BA was calculated from the largest activated cluster (contiguous activated voxel group) in the BA. Percent BOLD signal change outcome for the largest cluster in each region was measured as semantic encode and semantic recognition. Percent BOLD signal change for semantic encode for a specific BA was calculated as a contrast of (percent BOLD signal change for word encode) minus (percent BOLD signal change for pseudo-word encode). Similarly, percent BOLD signal change for semantic recognition was calculated as a contrast of (percent BOLD signal change for word recognition) minus (percent BOLD signal change for pseudo-word recognition). These contrasts were devised based on the fact that word encode/recognition and pseudo-word encode/recognition differed only according to the semantic aspects of the stimuli. As shown in Figure 1, scanner runs were 376 seconds consisting of a 20 second fixation (white cross on black screen) followed by an initial encode session (5 words or pseudo-words) displayed for 10 seconds, a fixation cross displayed for 12 seconds, and a recognition phase (10 seconds). Each trial consisted of 4 encoding and 4 recognition blocks for words and 4 encoding and 4 recognition blocks for pseudo-words. Subjects completed two trials (runs) of the task. The trials differed only by the random order of presentation of the encode and recognition events.
Behavioral Performance
Secondary outcome variables were response time for correct responses for word recognition or pseudo-word recognition. Responses were analyzed with regard to percentage correct responses, reaction time, or accuracy (number of omission and commission errors) for both the word and pseudo-word portions of the task. Because the behavioral measures do not permit a simple subtraction of effects as analyzed for the regional BOLD signal change data, we reported word recognition and pseudo-word recognition measures separately, and not as a semantic construct. As such, behavioral measures were analyzed in relation to BOLD signal change during word or pseudo-word recognition but not for the derived semantic measure. Of the 16 subjects completing imaging, behavioral data for the recognition phase was not obtained for 1 subject. Although prior reports have found no association between MDMA use and verbal intelligence (Jager et al. 2007;Jager et al. 2008;Schilt et al. 2007), we assessed verbal intelligence quotient (IQ) using the WASI-R in a subgroup of participants to examine for associations of task performance with IQ.
Data acquisition
Imaging
Echo planar images sensitive to BOLD signal changes were acquired on a 3.0 T General Electric™ whole body MR scanner during performance of the homophone task. We used the following scan parameters: flip angle 90 degrees, TE/TR 40/2000 ms, FOV 24 × 24 cm, 19 slices, slice thickness 5 mm, gap 0 mm, and a 64 × 64 imaging matrix with in-plane resolution of 3.75 mm.
Data pre-processing
Functional scans were analyzed in Brain Voyager QX (BVQX), Version 1.7 (Brain Innovation, Maastricht, The Netherlands). Data were preprocessed as follows: Three-dimensional motion correction by trilinear interpolation. Data having greater than 2 mm translational movement or 2 mm rotational movement was excluded. Linear trend removal and a 3 cycles/ time course high pass filter were employed. Scans were transformed into Talairach coordinate space (Talairach et al. 1988).
Intrasubject fMRI modeling
Using a Talairach BA atlas, images for regional BOLD signal time course analysis were masked to include only left BA 9, 18, 21/22 and 45. This was accomplished in BVQX by loading the Talairach atlas Daemon as a BA voxel of interest file and creating a mask file using the BA regions (Lancaster et al. 2000). Tasks were modeled as predictors in BVQX to screen data for activation in the regions of interest and to generate images for displaying activation. We used a False Discovery Rate (FDR) of 0.05 corrected for total voxels in the regions of interest to display the regional brain activation maps for figure 2 (Genovese et al. 2002). The time course was modeled using a two gamma hemodynamic response function (HRF) with onset 0, time to peak 5 s, response undershoot ratio of 7, time to undershoot peak of 15 s, response dispersion 1 and undershoot dispersion 1. Because we did not plan a case/control comparison, we did not employ a statistical cutoff to produce regional brain activation maps for analysis but instead analyzed the percent BOLD signal change during task performance for each subject individually as the contrast of word encode minus pseudo-word encode and word recognition minus pseudo-word recognition. Using BVQX, average time course percent BOLD signal change was computed for each region in each subject as [(BOLD signal during task – BOLD signal during baseline/BOLD signal during baseline) × 100]. The regional percent BOLD signal change data was then entered into the correlation analyses (see below).
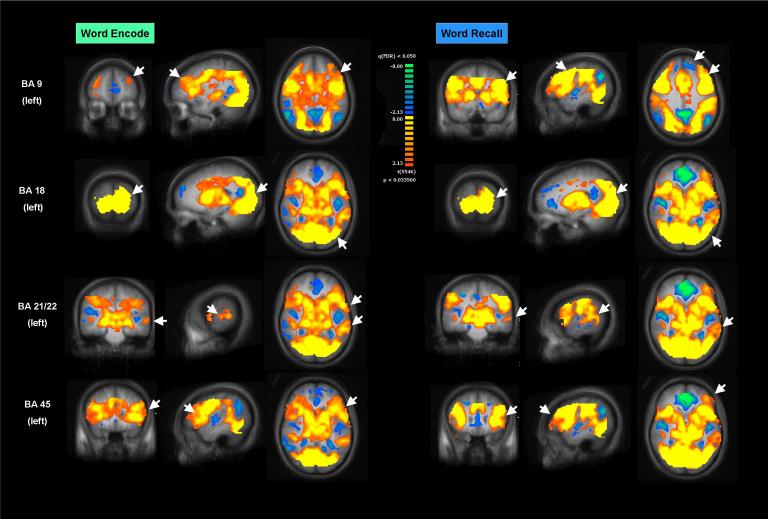
Figure 2. Activation to Word Encode and Word Recognition.
Task-induced regional brain activation maps for word encode and word recognition. Maps were thresholded at a false discovery rate (FDR) of < 0.05 (see methods for details). Rows show (top to bottom) regional brain activation to Brodmann Area (BA) 9, 18, 21/22 and 45. White arrowheads point to BA in each brain view. For each condition (word encode or word recognition) activation maps are overlayed on a group average structural brain in (left to right) coronal, sagittal, and axial sections. Right side of figure is left side of brain for coronal and axial sections. Right side is posterior for sagittal sections. Scale bar indicates t values ranging from −8.00 to plus 8.00. Warm colors are positive t, indicating increased activation with task activity. Cool colors are negative t, indicating decreased activation with task activity. All regions of interest used for analysis showed increased task-induced activation.
Statistical analysis
Outcome measures of percent BOLD signal change for each area of interest, number of correct responses, number of omission errors, number of commission errors and response time (for correct responses) for recognition were exported to SPSS for statistical analysis (SPSS for Windows version 15.0 software; SPSS Inc.). Continuous variables were analyzed using Spearman’s test. Results were considered statistically significant on 2-tailed analysis if p < 0.05. Results presented are means and standard deviations unless otherwise specified.
RESULTS
Demographics
All subjects were right-handed. 10 were male and 6 were female. 1 subject was black, 1 was Asian, 1 was Asian/Caucasian and all other participants were Caucasian. Age was 23.6 ± 2.7 years (range 20-29 years) and Wechsler Abbreviated Scale of Intelligence (WASI) verbal intelligence score was 60.2 ± 7.4 (range 50-71). WASI verbal IQ was within normal range and consistent with verbal IQ scores previously recorded in MDMA users and controls (Zakzanis et al. 2002).
Drug exposure history
Lifetime drug use exposure as episodes and units is summarized in table 1 as mean, standard deviation of the mean (S.D.) and minimum and maximum for subjects reporting exposure to a specific drug. Twelve of our total cohort of 16 polysubstance users used MDMA. All MDMA users also used cannabis and alcohol. Ten subjects used cocaine, of whom 8 were MDMA users. Six subjects used methamphetamine, of whom 5 were MDMA users. But for 3 subjects who had used codeine, there was no reported use of opioids in our cohort. There were statistically significant correlations between lifetime episodes of MDMA use and lifetime episodes of use of cannabis (rs=0.752; p=0.001), cocaine (rs=0.666; p=0.005), LSD (rs=0.686; p=0.003) and methamphetamine (rs=0.750; p=0.001). There were no statistically significant correlations between lifetime episodes of MDMA and lifetime episodes of use of alcohol (rs=0.094; p=0.739) or psilocybin (rs=0.465; p=0.069).
Table 1.
Lifetime Substance Use
| Drug | n | Episodes Use |
Quantity Use |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std. Dev | Min | Max | Mean | Std. Dev | Min | Max | ||
| MDMA (mg) | 12 | 43.33 | 40.73 | 8 | 155 | 4607.08 | 4131.24 | 750.0 | 15,325 |
| Alcohol (units) | 15 | 548.73 | 751.75 | 4 | 2,110 | 1761.60 | 2721.14 | 4.0 | 10,171 |
| Cannabis (joints) | 12 | 726.33 | 710.38 | 9 | 2,000 | 2092.56 | 3725.10 | 8.0 | 12,904 |
| Cocaine (mg) | 9* | 46.60 | 124.31 | 1 | 400 | 7.17 | 14.74 | 0.3 | 46 |
| LSD (mcg) | 9 | 24.89 | 21.77 | 4 | 65 | 5758.33 | 6765.29 | 295.0 | 18,700 |
| Psilocybin (mg) | 8 | 9.38 | 6.78 | 2 | 20 | 32.69 | 28.64 | 2.0 | 75 |
| Meth (mg ) | 7 | 56.43 | 63.68 | 1 | 166 | 2468.57 | 2989.58 | 40.0 | 8,110 |
Data missing from one subject
LSD = Lysergic acid diethylamide
Meth = Methamphetamine
The length of abstinence from substance use prior to starting the study is shown in table 2. All MDMA users were abstinent from MDMA for over 2 weeks. One subject in the cannabis cohort used 1 day prior to the study while all other users reported no use for at least 4 days. Cocaine users had been abstinent for at least 16 days. Methamphetamine use was remote with reported last use at least 157 days ago.
Table 2.
Abstinence from Drug Use
| Drug | n | Mean days since last use |
Std. Dev |
Min | Max |
|---|---|---|---|---|---|
| MDMA | 11 | 476.55 | 326.28 | 19 | 1132 |
| Alcohol | 16 | 32.19 | 68.87 | 1 | 274 |
| Cannabis | 12 | 75.33 | 136.17 | 1 | 385 |
| Cocaine | 10 | 234.4 | 283.87 | 16 | 764 |
| LSD | 8 | 897.63 | 772.31 | 110 | 2227 |
| Psilocybin | 7 | 718.71 | 649.71 | 60 | 1862 |
| Meth | 6 | 875.17 | 759.75 | 157 | 2224 |
LSD = Lysergic acid diethylamide
Meth = methamphetamine
Drug use and regional brain activation
Overall task effects
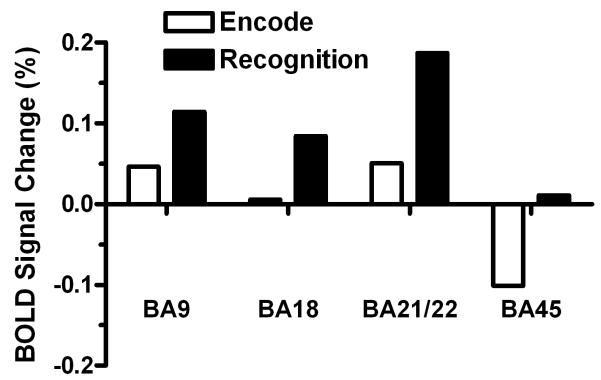
As seen in figure 2, the word encode and word recall tasks produced robust and widespread regional brain activation at the selected statistical threshold (FDR = 0.05). More specifically, the task activated left BA 9, 18, 21/22 and 45 in both conditions. A similar pattern was seen for the pseudo-encode and pseudo-recall task (not shown). The analysis of percent BOLD signal change for semantic encode or recognition (defined as percent signal change during word task-percent signal change during pseudoword task) revealed that our task produced net positive activations for semantic encode and recognition in BA 9, 18, 21/22. For BA 45, the task produced negative BOLD signal change for semantic encode and positive BOLD signal change for semantic recognition (Figure 3).
Figure 3. Regional Semantic percent BOLD signal change.
Bar chart indicating regional semantic percent BOLD signal change as the percent signal change in a region of interest for word encode or recognition minus pseudo-word encode or recognition.
Semantic Encode
For the semantic encode task, there was no association between lifetime MDMA use and percentage BOLD signal change for left BA 9, BA 18, BA 21/22 or BA 45 (Table 3—all correlations are calculated within the group exposed to a particular drug). For the other most commonly used drugs (alcohol, cannabis, methamphetamine, LSD, psilocybin), there were no significant associations between drug use and percent BOLD signal change during semantic encode for BA 18 or BA 21/22. For BA 9, there was a significant negative correlation between lifetime methamphetamine use and percent BOLD signal change (rs=−0.786; p=0.036). For BA 45, only lifetime episodes of alcohol use showed a significantly positive association with percent BOLD signal change (rs=0.518; p=0.048).
Table 3.
Brain Activation and Lifetime Drug use Correlations
| Semantic Encode |
Semantic Recognition |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lifetime Episodes |
n | BA 9 | BA 18 | BA 21/22 |
BA 45 | BA 9 | BA 18 | BA 21/22 |
BA 45 |
| r | r | r | r | r | r | r | r | ||
| P | P | P | P | P | P | P | P | ||
| MDMA | 12 | −0.028 | -0.169 | −0.070 | −0.035 | −0.789 | −0.606 | −0.683 | −0.324 |
| 0.931 | 0.599 | 0.828 | 0.913 | 0.002 | 0.037 | 0.014 | 0.304 | ||
|
| |||||||||
| Alcohol | 15 | 0.379 | 0.250 | −0.098 | 0.518 | 0.004 | −0.250 | −0.121 | −0.214 |
| 0.164 | 0.369 | 0.727 | 0.048 | 0.990 | 0.369 | 0.666 | 0.443 | ||
|
| |||||||||
| Cannabis | 12 | 0.147 | −0.189 | 0.217 | 0.308 | −0.580 | −0.531 | −0.182 | −0.483 |
| 0.649 | 0.557 | 0.499 | 0.331 | 0.048 | 0.075 | 0.572 | 0.112 | ||
|
| |||||||||
| Cocaine | 10 | 0.310 | −0.170 | 0.103 | 0.413 | −0.851 | −0.669 | −0.608 | −0.571 |
| 0.383 | 0.638 | 0.776 | 0.235 | 0.002 | 0.035 | 0.062 | 0.084 | ||
|
| |||||||||
| LSD | 9 | −0.183 | −0.517 | −0.067 | −0.383 | −0.083 | 0.300 | 0.167 | −0.233 |
| 0.637 | 0.154 | 0.865 | 0.308 | 0.831 | 0.433 | 0.668 | 0.546 | ||
|
| |||||||||
| Psilocybin | 8 | −0.263 | −0.707 | −0.563 | −0.299 | −0.299 | 0.311 | 0.192 | −0.347 |
| 0.528 | 0.050 | 0.146 | 0.471 | 0.471 | 0.453 | 0.649 | 0.399 | ||
|
| |||||||||
| Meth | 7 | −0.786 | 0.464 | 0.464 | −0.571 | −0.357 | −0.464 | −0.464 | −0.214 |
| 0.036 | 0.294 | 0.294 | 0.180 | 0.432 | 0.294 | 0.294 | 0.645 | ||
LSD = Lysergic acid diethylamide
Meth = methamphetamine
Semantic Recognition
Within the MDMA-exposed group there were statistically significant negative correlations between MDMA use and percent BOLD signal change in left BA 9, 18 and 21/22, but not BA 45 (Table 3). For BA 9, both lifetime episodes of MDMA use and lifetime milligrams of MDMA use showed a statistically significant inverse association with percent BOLD signal change. Only “lifetime episodes of MDMA use” was statistically significantly inversely associated with percent BOLD signal change in BA 18 and 21/22.
Within the cannabis-exposed cohort, there was a significant negative correlation between lifetime episodes of use and percent BOLD signal change in left BA 9 (rs=−0.580; p=0.048), and for lifetime joints used and percent BOLD signal change in left BA 45 (rs=−0.587; p=0.045). After controlling for cannabis use in the covariate analysis, lifetime episodes of MDMA use remained statistically significantly negatively associated with percent BOLD signal change in left BA 9 (rs=−0.680; p=0.021).
Within the cocaine-exposed group, cocaine exposure was inversely associated with percent BOLD signal change in left BA 9 and 18 during semantic recognition. This was true for both lifetime episodes of use (BA 9: rs=−0.851, p=0.002; BA 18: rs=−0.669, p=0.035) and lifetime grams of cocaine used (BA 9: rs=−0.683, p=0.042; BA 18: rs=−0.767, p=0.019). The negative correlation between lifetime episodes of MDMA use and BA 9 remained significant after controlling for the association of BA 9 with lifetime episodes of cocaine (rs=−0.669, p=0.049). After controlling for lifetime episodes of cocaine use, the negative correlation between lifetime episodes of MDMA and percent BOLD signal change in left BA 18 no longer achieved statistical significance (rs=−0.534, p=0.139).
For all other most commonly used drugs (cohorts with exposure to alcohol, methamphetamine, LSD or psilocybin), there was no statistically significant association between substance exposure and BOLD activation during semantic recognition.
Abstinence
There was a statistically significant negative correlation between days since last use of MDMA and activation in BA 18 for semantic recall (rs=−0.700, p=0.016). There were also statistically significant negative correlations between days since last use of cannabis and regional brain activation in BA 45 for semantic encode (rs=−0.715, p=0.009), and for semantic recall (rs=−0.753, p=0.005).
Drug use and task performance
For the subgroup of subjects having WASI verbal IQ scores, there was no statistically significant association of WASI score with lifetime episodes (rs=−0.186, p=0.631, n=9) or milligrams (rs=−0.267, p=0.488, n=9) of MDMA use, or between WASI score and regional brain activation in left BA 9, 18, 21/22 or 45 for semantic encoding or semantic recognition. Further, there was no association of WASI score with recognition task performance measures (percent correct responses, reaction time or accuracy [number of omission and commission errors] for both the word and pseudo-word portions of the task).
For the MDMA users, there was no significant association between lifetime episodes or milligrams of MDMA use and the recognition task performance measures (table 4—all correlations are calculated within the group exposed to a particular drug).
Table 4.
Lifetime Episodes of Drug Use and Semantic Task Performance Correlations
| Word |
Pseudoword |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Percent Correct |
Reaction Time (ms) |
Omission (n) |
Commission (n) |
Percent Correct |
Reaction Time (ms) |
Omission (n) |
Commission (n) |
||
| Lifetime Episodes |
n | r | r | r | r | r | r | r | r |
| P | P | P | P | P | P | P | P | ||
| MDMA | 9 | 0.051 | −0.502 | 0.176 | 0.145 | 0.051 | 0.259 | 0.061 | −0.184 |
| 0.896 | 0.168 | 0.651 | 0.710 | 0.897 | 0.500 | 0.877 | 0.635 | ||
|
| |||||||||
| Alcohol | 13 | 0.352 | −0.352 | −0.630 | −0.271 | −0.171 | −0.203 | 0.356 | −0.118 |
| 0.239 | 0.239 | 0.838 | 0.370 | 0.576 | 0.505 | 0.233 | 0.701 | ||
|
| |||||||||
| Cannabis | 9 | 0.458 | −0.767 | −0.096 | −0.076 | 0.580 | −0.333 | −0.397 | −0.411 |
| 0.215 | 0.016 | 0.806 | 0.845 | 0.102 | 0.381 | 0.290 | 0.272 | ||
|
| |||||||||
| Cocaine | 8 | 0.311 | −0.452 | 0.061 | −0.436 | −0.132 | −0.143 | 0.331 | −0.358 |
| 0.453 | 0.260 | 0.885 | 0.280 | 0.756 | 0.736 | 0.423 | 0.385 | ||
|
| |||||||||
| LSD | 7 | 0.074 | 0.250 | 0.487 | −0.436 | 0.309 | −0.071 | 0.154 | −0.468 |
| 0.875 | 0.589 | 0.268 | 0.328 | 0.500 | 0.879 | 0.741 | 0.290 | ||
|
| |||||||||
| Psilocybin | 7 | 0.150 | −0.360 | 0.663 | −0.422 | 0.587 | −0.414 | −0.156 | −0.577 |
| 0.749 | 0.939 | 0.104 | 0.346 | 0.166 | 0.355 | 0.739 | 0.175 | ||
|
| |||||||||
| Meth | 6 | −0.406 | −0.486 | 0.213 | 0.493 | 0.143 | 0.371 | −0.334 | −0.031 |
| 0.425 | 0.329 | 0.686 | 0.321 | 0.787 | 0.468 | 0.518 | 0.954 | ||
LSD = Lysergic acid diethylamide
Meth = methamphetamine
For the cannabis-exposed group, there was a statistically significant negative correlation between lifetime cannabis exposure and reaction time on the word portion of the recognition task (table 4). This was true for both episodes of use (rs=−0.767, p=0.016), and number of joints used (rs=−0.700, p=0.036). Further, there was a significant negative correlation between lifetime joints of cannabis and accuracy (number of commission errors) on the pseudo-word portion of the recognition task (rs=−0.691, p=0.039).
For psilocybin-exposed subjects, there was a significant positive association between lifetime grams of psilocybin used and percent correct responses on the pseudo-word portion of the recognition task (rs=0.855, p=0.014).
Across the full cohort of subjects there was no significant association between the recognition task performance measures and regional brain activation during word and pseudo-word recognition for left BA 9, 18, 21/22 or 45. Because we used a block design for the fMRI task we could not isolate individual trials to segregate brain activation by correct/incorrect trial.
DISCUSSION
Interpretation of Results
In this cohort of polysubstance users, lifetime MDMA exposure was statistically significantly associated with reduced brain activation during a semantic recognition task across multiple Brodmann regions (left BA 9, 18 and 21/22). MDMA use was not statistically significantly associated with regional brain activation during semantic encoding and there were no clear patterns of effects from other drugs. The association of reduced regional brain activation in left BA 18 and 21/22 with MDMA use is consistent with Cowan et al.’s previous report of reduced brain gray matter concentration in the same regions in a cohort of MDMA polydrug users (there was no overlap between participants in the previous and current report) (Cowan et al. 2003). The finding of reduced brain activation in left BA 18 and 21/22 may suggest a locus for brain regions mediating the well-established reduction in aspects of verbal memory in MDMA users. The finding of an MDMA effect in the recognition but not the encoding phase suggests that verbal memory may be impaired at the level of recognition, and not encoding. This is consistent with our prediction that a structural reduction in brain gray matter would be associated with functionally altered regional brain activation during verbal semantic recognition.
In our cohort of polysubstance users with a history of moderate lifetime MDMA exposure (according to the criteria of Fox et al.) (Fox et al. 2001), lifetime episodes of MDMA and cocaine use were not associated with altered performance on the verbal recognition task (as measured by word recognition reaction time or number of omission and commission errors), while lifetime cannabis use was associated with shorter word recognition reaction time and worse accuracy (increased commission errors). This contrasts with previous studies that have found evidence of verbal memory impairment in MDMA users (Laws et al. 2007;Reneman et al. 2001b;Schilt et al. 2007). Possible interpretations include lifetime MDMA exposure in our cohort being too low to result in significant impairment in task performance (Parrott 2006;Reneman et al. 2001b), confounding by use of other substances, small sample size, or neurocognitive deficits that our verbal recognition task lacked sensitivity to detect. The relationship between BOLD signal and task performance is difficult to predict. However, it is important to note that there was an inverse relationship between MDMA use and BOLD signal change for brain regions that are implicated in semantic processing.
Task
Our paradigm intended to distinguish brain activation attributable to semantic memory. It is important to recognize that task limitations precluded complete isolation of semantic memory. As shown in figure 3, differences in percent BOLD signal change for the semantic contrast were generally small. This may be due to the fact that much of the task-related activation was due to non-specific aspects of working memory or because the percent BOLD signal change was averaged over an entire Brodmann Area, which may not have exact correspondence to the portion of the region of interest relevant to semantic processing. Working memory was significantly represented in our paradigm due to the relatively short duration of time (10 seconds) between presentation of the words and onset of the forced choice task (Linden 2007). This is likely to have contributed to the activation observed in BA 9 and 45 in particular, which are both significantly associated with working memory function in addition to their roles in semantic recognition. It is also possible that several pseudo-words may have been adequately reminiscent of English words to result in limited semantic activation in some subjects during the pseudo-word portion of the task.
Polysubstance Use
Lifetime cocaine exposure was associated with reduced regional brain activation in left BA 9 and 18, while lifetime cannabis use was associated with reduced brain activation in left BA 9 during semantic recognition. After controlling for lifetime exposure to cannabis and cocaine, the association of MDMA use with BA 9 activation remained significant. However the association between MDMA exposure and brain activation in BA 18 was no longer significant on controlling for lifetime cocaine use.
Use of cocaine was reported in 8 of our cohort of 12 MDMA polysubstance users. Several studies have reported impaired delayed and short-term verbal memory in chronic cocaine users compared to controls (Ardila et al. 1991;Mittenberg et al. 1993;Pace-Schott et al. 2008). This may have confounded the association of lifetime MDMA use with reduced left BA 18 (occipital cortex) activation since Tomasi et al. have reported increased BOLD activation in the occipital cortex of cocaine users during a working memory task (Tomasi et al. 2007). The association of cocaine exposure with increased brain activation in the occipital cortex contrasts with the present study’s finding of the association of MDMA exposure with reduced brain activation in left BA 18. This suggests that MDMA exposure is likely to be a primary driver of the association for cocaine and MDMA with reduced regional brain activity in left BA 18.
A history of significant methamphetamine use was reported in 7 of the 12 MDMA users (56.4±63.7 lifetime episodes of use). Use was typically remote (875.2±759.8 days since last dose). There was a statistically significant negative correlation between lifetime episodes of methamphetamine use and percent BOLD signal change in left BA 9 for semantic encode (rs=−0.786, p=0.036) but not for the semantic recall portion of the task. There were no associations between lifetime use of any other drugs and regional brain activation for semantic encoding. There were no correlations between methamphetamine use and task performance measures. Neuropsychological studies have demonstrated deficits in explicit memory, attention and selective inhibition in methamphetamine users. Our findings are consistent with several functional studies showing reduced activation in brain regions involved in semantic and working memory processing in methamphetamine-dependent subjects. Paulus et al. found reduced activation in parietal cortical regions (precuneus and post-central gyrus) in methamphetamine-dependent subjects participating in a decision-making task (Paulus et al. 2003). The same group found that methamphetamine-dependent subjects showed reduced activation of dorsolateral prefrontal cortex (BA 9) during performance of a prediction task (Paulus et al. 2002). Relapsed methamphetamine-dependent subjects also showed hypoperfusion in the left superior temporal gyrus (BA 21/22), a region involved in lexical-semantic processing (Paulus et al. 2005).
Every MDMA polysubstance user in our cohort had a history of cannabis use, which has been commonly reported in MDMA users and represents a significant potential confounder (Gouzoulis-Mayfrank et al. 2006;Parrott 2006). The statistically significant negative correlations between days since last use of cannabis and regional brain activation in BA 45 for semantic encode and semantic recall is consistent with literature supporting reduced subacute brain activation in recently abstinent cannabis users, particularly in the prefrontal cortex. Several neuropsychological studies have shown that cannabis use may have chronic negative effects on short-term memory, including working and episodic memory (Bolla et al. 2002;Pope, Jr. et al. 2001;Solowij et al. 2002;Yucel et al. 2008). Herning et al. found increased resistance of cerebral vessels during cannabis withdrawal (Herning et al. 2005). Several functional studies in recently abstinent chronic cannabis users have shown decreased regional cerebral blood flow in prefrontal cortex at rest (Sneider et al. 2008) and during performance of a verbal memory task (Block et al. 2002). Using PET, Block et al. found a corresponding decrease in brain activation in memory-associated regions during performance of a word recall task in recently abstinent cannabis users (Block et al. 2002). Conversely, some studies have found evidence of increased activation (Chang et al. 2006). Our analysis showed that the relationship between lifetime MDMA exposure and reduced activation in BA 9 during semantic recognition remained significant when controlling for cannabis exposure. Cannabis use did not show as widespread or consistent a negative correlation with brain activation as MDMA use. This suggests that the observed statistical findings may be more determined by the strong correlation of MDMA use with cannabis use rather than with specific effects of cannabis.
Neocortex
Brain regions that have been associated with semantic processing include left temporal cortex (BA 21/22) and left parietal cortex (Friedman et al. 1998). BA 18 is located in occipital cortex and is involved in visual learning involving name-face recognition (Herholz et al. 2001). Left BA 21 is involved in semantic memory retrieval and higher level processing of meaning (Booth et al. 2002;Chou et al. 2006;Lee et al. 2002). Left BA 22, which was confluently activated with BA 21 during our fMRI paradigm, corresponds to left superior temporal gyrus. It is involved in converting sensory input into recognized word forms (Petersen et al. 1988), which was an integral requirement in our task.
Left BA 45 is activated during tasks involving semantic memory retrieval (Booth et al. 2002;Lee et al. 2002), semantic fluency (Amunts et al. 2004), verbal working memory (Wager et al. 2005) and its activation is greater during more demanding semantic tasks (Chou et al. 2006). However, the role of BA 45 in these tasks may be related to a more general executive control process rather than specific to semantic processing. While we did not find a relationship between MDMA exposure and activation in left BA 45, there was a significant relationship between lifetime joints of cannabis use and reduced brain activation in this region.
Left BA 9 corresponds to the dorsolateral prefrontal cortical region of the frontal lobe (Fitzgerald et al. 2006). It is associated with central executive function including attentional selection, impulsivity, decision making, organization, monitoring of memory stimuli (Baxter et al. 2008;Robbins 2005), and also shows significant involvement in working memory function (Funahashi 2006; Babiloni et al. 2005;Wager et al. 2005). This region was strongly activated by our fMRI task. During the present study, lifetime use of MDMA, cannabis and cocaine were each associated with reduced brain activation in this region, and the association with MDMA exposure remained statistically significant when controlling for cannabis and cocaine exposure. In contrast to our findings of reduced left BA 9 activation, Moeller et al. found greater BOLD activation in prefrontal cortex in MDMA users compared to controls during a delayed working memory task (Moeller et al. 2004). This difference may be secondary to the different tasks employed in the two studies or to other experimental or cohort effects.
Implications of altered regional brain activation
The association between the degree of MDMA exposure and reduced activation in left BA 9, 18 and 21/22 permits several possible explanations regarding the neural origins of this finding. While the finding of a consistent exposure-response relationship involving multiple regions involved in semantic recognition is suggestive of causation, there are several other potential interpretations of this finding. First, since there were associations between regional brain activation and exposure to MDMA, cannabis, cocaine and methamphetamine, it is possible that the current results are related to pre-existing brain differences that predispose to increased polydrug use or increased likelihood for MDMA consumption. However, the correlation between degree of drug use and outcomes was most consistent in the regions of interest for MDMA effects, which does not support a predisposition to drug use in general. Second the regional brain activation could be reflective of a general effect of drug exposure. However, the fact that MDMA effects remained significant after controlling for cannabis and cocaine use in left BA 9 suggests that there may be a specific effect of MDMA. Third, other unknown factors not assayed or controlled for in this study could independently correlate with MDMA use and with regional brain activation. Fourth, although altered regional brain activation suggests that neural function is altered, the direction of altered brain activation (i.e. increased or decreased) does not permit conclusions regarding the pathological implications of the observed finding. Namely, both increases and decreases in regional brain activation have been associated with pathological processes (Bondi et al. 2005). Present evidence suggests that activation in a brain region is most strongly determined by synaptic input to a brain region and secondarily by local synaptic processing in the brain region, but not by neuronal firing in the activated region (Rauch et al. 2008). Other neurophysiological events may be largely undetectable by the BOLD method except as a downstream consequence of their effects on synaptic transmission (Logothetis et al. 2004). This suggests that reduced activation in a brain region may be more dependent upon alterations in synaptic inputs to that region, rather than a function of structural or functional alterations in the region of study. Because this study was primarily aimed at probing brain activation, we did not administer neuropsychological testing of verbal memory encoding and recognition separately from the tasks performed in the scanner. However, we did obtain verbal IQ assessments (WASI) and reaction time and accuracy measures for the recognition phase of the task. WASI scores did not show a correlation with MDMA exposure or performance on the verbal recognition task.
Neural basis of the observed findings
Findings from basic science investigations of MDMA effects provide a framework for interpreting the current results. Cowan et al. (Cowan et al. 2008) have previously outlined a cortical model or framework for interpreting neuroimaging studies in human MDMA users. Essential features of the model relevant to the current report are: 1) MDMA exposure is associated with persisting changes in serotonergic neurotransmission, 2) changes in serotonergic neurotransmission may be evident in loss of coupling between serotonin and brain neurotrophic factors (resulting in regional brain gray matter shrinkage), 3) changes in serotonergic neurotransmission may be evident in altered cell-cell synaptic signaling irrespective of structural neuronal changes. As such, structural or functional effects of reduced 5-HT neurotransmission may contribute to the observed findings.
Initial studies of MDMA administration in animals suggested that MDMA produced a fine-diameter axotomy of 5-HT neurons with sparing of brainstem cell bodies (Green et al. 2003;Kish 2002). More recent animal studies have questioned this finding, suggesting that at doses potentially closely mimicking those of human recreational users, MDMA does not produce axotomy and loss of 5-HT markers does not necessarily indicate axotomy (Fantegrossi et al. 2004;Wang et al. 2004;Wang et al. 2005). One widely-studied marker for 5-HT system integrity is the serotonin reuptake transporter (5-HTT). The 5-HTT is present on serotonergic axons and is the primary molecular structure controlling the duration of post-release 5-HT signaling. A reduction in 5-HTT levels following MDMA exposure is consistent either with serotonergic axotomy or with down-regulation of transporter expression. Studies of 5-HTT binding have commonly demonstrated reductions in 5-HTT levels in human MDMA users with some studies suggesting gender effects and others suggesting potential transporter recovery with increased duration of MDMA abstinence (de Win et al. 2004;McCann et al. 1998;McCann et al. 2005;McCann et al. 2008;Reneman et al. 2001a;Semple et al. 1999); Buchert et al. 2004; Thomasius et al. 2003). Additional study is needed before the specific time period and degree of recovery is fully clarified. A study examining 5-HT2A receptor expression in human MDMA users reported upregulation of these postsynaptic receptors in abstinent users, which is consistent with, but not confirmatory of, long-lasting reductions in the concentration of presynaptic 5-HT (Reneman et al. 2002).
5-HT depletion by MDMA could play an important role in the verbal memory deficits observed in human MDMA users and the reduced activation in left BA 9, 18 and 21/22 observed in the present study. Using tryptophan depletion to acutely lower 5-HT levels, Allen et al. found increased BOLD activation in the left posterior cingulate cortex and reduced activation in the superior frontal gyrus and left precuneus during performance of a 2-back verbal working memory task. This suggested that 5-HT is involved in modulation of pre-frontal engagement during verbal working memory functioning (Allen et al. 2006). Using fMRI to study subjects taking a serotonin selective reuptake inhibitor (SSRI) during performance of an N-back working memory task, Rose et al. found increased left inferior frontal gyrus (BA 45) activation (Rose et al. 2006). While it is tempting to speculate that the latter study supports the association of a 5-HT rich state with increased BOLD signal in a brain region involved in verbal working memory, caution must be used in extrapolating these findings to the effects of MDMA on the serotonin system and verbal memory. Of note, BA 45 was the one area studied where we found no statistically significant association of MDMA use with task-evoked BOLD signal. While tryptophan depletion represents an acute state of 5-HT depletion, MDMA use is associated with more chronic serotonergic changes and possible adaptive neuromodulatory responses. Secondly, SSRIs are not completely selective in their effects on the serotonin system and their chronic effects are likely to induce significant adaptive changes (Butler et al. 2008). Clearly 5-HT has a complicated role in memory function (Schmitt et al. 2006).
Limitations
Several important limitations to this study restrict our ability to conclude whether or not MDMA-associated neurotoxicity is responsible for the observed findings. First, the cross-sectional correlational design of the study does not permit conclusions regarding cause and effect. It is possible that genetic or other influences pre-disposing to MDMA polydrug use may also be associated with pre-existing brain changes. Second, it is possible that a larger sample size may have resulted in some non-significant trends reaching statistical significance. Further, because regional semantic percent BOLD signal change was generally small (figure 3), this could have resulted in failure to find correlations between MDMA use and activation in some cases. Third, the degree of polydrug exposure seen worldwide in MDMA users precludes the ability to recruit a well-matched control group (van Reekum et al. 2001). MDMA users tend to use more of every class of drug than do their non-MDMA using peers (de Almeida et al. 2003;Scholey et al. 2004;Wish et al. 2006) and polydrug use can particularly confound case control designs when a class of drug is strongly correlated with MDMA use or when associated with outcome measures. In a functional neuroimaging report of visual system activation, MDMA users and controls did not differ in degree of mean regional brain activation while MDMA use (but not other drugs) was positively correlated with activation measures (Cowan et al. 2007). This suggested that other drug exposure may have affected the mean activation in the MDMA group. Therefore, within group designs assaying for correlations between the degree of MDMA exposure and outcome variables may have specific utility in the study of brain function in human MDMA-exposed cohorts. Fourth, in addition to testing our primary hypothesis, we performed numerous exploratory correlational analyses examining the association of drug exposure and percent BOLD signal change in 3 brain regions across two tasks. Because increases or decreases in percent BOLD signal change in a brain region may be associated with functional decrements, this hypothesis required a two-tailed test for significance. Because of the exploratory nature of the additional comparisons, we did not employ correction for multiple independent statistical comparisons. As such, it is possible that some of our findings were due to type I error related to multiple comparisons. Fifth, because VBM studies of the type previously reported (Cowan et al. 2003) require a much larger cohort than presented here, we do not yet know if the current group of MDMA users show regional brain gray matter changes in the regions previously reported by Cowan et al. (Cowan et al. 2003). Our preliminary analyses (unpublished observations) of a larger cohort that includes members from the present study is not consistent with structural effects in these regions. Data collection for a VBM study that will include the current cohort is ongoing and larger longitudinal studies, such as those of the Netherlands XTC project (de Win et al. 2005), are necessary to more fully address these confounds.
Because we employed an a-priori hypothesis-based region of interest analysis, we did not explore additional brain regions potentially involved in aspects of memory encoding and retrieval. Others have speculated that altered hippocampal structure and function may be related to impaired memory encoding in MDMA users (Daumann et al. 2005;Jacobsen et al. 2004). Specific tasks designed to probe hippocampal function in MDMA users are needed to address this topic. As reviewed (Cowan 2007), there is presently little overlap between various imaging modalities and their outcomes with regard to studies of MDMA polydrug users.
Conclusions
Using an fMRI semantic memory paradigm in a cross-section of polysubstance users enriched for MDMA use, this study found an association between lifetime MDMA use and reduced BOLD activation in left BA 9, 18 and 21/22 but not 45 during semantic recognition. There was no significant association between MDMA exposure and performance time or accuracy on the semantic recognition task.
The finding of reduced brain activation in left BA 9, 18 and 21/22 suggests a potential locus for brain regions mediating the well-established reduction in aspects of verbal memory in MDMA users. The association of reduced brain activation with lifetime MDMA use is suggestive of a possible causal role for MDMA in effecting functional changes in brain regions involved in verbal memory processing. Further investigation is required to replicate and elaborate on these preliminary findings.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the technical assistance of Linda Todd, Emre Genca, Eiman Shafa and Kimberly L. Morton. We also thank Dr. Lee for his advice regarding the fMRI paradigm. We would like to thank the following funding agencies: NIDA: DA015137, DA020149 and DA00366; NCRR: Vanderbilt CTSA UL1 RR024975; and VUIIS: Vanderbilt University Institute of Imaging Science.
References
- Allen PP, Cleare AJ, Lee F, Fusar-Poli P, Tunstall N, Fu CH, Brammer MJ, McGuire PK. Effect of acute tryptophan depletion on pre-frontal engagement. Psychopharmacology (Berl) 2006;187:486–497. doi: 10.1007/s00213-006-0444-x. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space--the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int.J Neurosci. 1991;57:73–79. doi: 10.3109/00207459109150348. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Ferretti A, Del GC, Carducci F, Vecchio F, Romani GL, Rossini PM. Human cortical responses during one-bit delayed-response tasks: an fMRI study. Brain Res.Bull. 2005;65:383–390. doi: 10.1016/j.brainresbull.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Back-Madruga C, Boone KB, Chang L, Grob CS, Lee A, Nations H, Poland RE. Neuropsychological effects of 3,4-methylenedioxymethamphetamine (MDMA or ecstasy) in recreational users. Clin Neuropsychol. 2003;17:446–459. doi: 10.1076/clin.17.4.446.27939. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Dorsolateral prefrontal lesions do not impair tests of scene learning and decision-making that require frontal-temporal interaction. Eur.J Neurosci. 2008;28:491–499. doi: 10.1111/j.1460-9568.2008.06353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Ponto L L Boles, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol.Biochem.Behav. 2002;72:237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, McCann UD, Ricaurte GA. Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology. 1998;51:1532–1537. doi: 10.1212/wnl.51.6.1532. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Hum.Brain Mapp. 2002;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert R, Obrocki J, Thomasius R, Vaterlein O, Petersen K, Jenicke L, Bohuslavizki KH, Clausen M. Long-term effects of ‘ecstasy’ abuse on the human brain studied by FDG PET. Nucl.Med Commun. 2001;22:889–897. doi: 10.1097/00006231-200108000-00007. [DOI] [PubMed] [Google Scholar]
- Butler SG, Meegan MJ. Recent developments in the design of anti-depressive therapies: targeting the serotonin transporter. Curr Med.Chem. 2008;15:1737–1761. doi: 10.2174/092986708784872357. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Bitan T, Burman DD, Bigio JD, Cone NE, Lu D, Cao F. Developmental and skill effects on the neural correlates of semantic processing to visually presented words. Hum.Brain Mapp. 2006;27:915–924. doi: 10.1002/hbm.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology (Berl) 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Bolo NR, Dietrich M, Haga E, Lukas SE, Renshaw PF. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007;155:179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Lyoo IK, Sung SM, Ahn KH, Kim MJ, Hwang J, Haga E, Vimal RL, Lukas SE, Renshaw PF. Reduced cortical gray matter density in human MDMA (Ecstasy) users: a voxel-based morphometry study. Drug Alcohol Depend. 2003;72:225–235. doi: 10.1016/j.drugalcdep.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Curran HV, Verheyden SL. Altered response to tryptophan supplementation after long-term abstention from MDMA (ecstasy) is highly correlated with human memory function. Psychopharmacology (Berl) 2003;169:91–103. doi: 10.1007/s00213-003-1463-5. [DOI] [PubMed] [Google Scholar]
- Daumann J, Fimm B, Willmes K, Thron A, Gouzoulis-Mayfrank E. Cerebral activation in abstinent ecstasy (MDMA) users during a working memory task: a functional magnetic resonance imaging (fMRI) study. Brain Res.Cogn Brain Res. 2003;16:479–487. doi: 10.1016/s0926-6410(03)00075-2. [DOI] [PubMed] [Google Scholar]
- Daumann J, Fischermann T, Heekeren K, Henke K, Thron A, Gouzoulis-Mayfrank E. Memory-related hippocampal dysfunction in poly-drug ecstasy (3,4-methylenedioxymethamphetamine) users. Psychopharmacology (Berl) 2005;180:607–611. doi: 10.1007/s00213-004-2002-8. [DOI] [PubMed] [Google Scholar]
- Daumann J, Jr., Fischermann T, Heekeren K, Thron A, Gouzoulis-Mayfrank E. Neural mechanisms of working memory in ecstasy (MDMA) users who continue or discontinue ecstasy and amphetamine use: evidence from an 18-month longitudinal functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:349–355. doi: 10.1016/j.biopsych.2004.06.011. [DOI] [PubMed] [Google Scholar]
- de Almeida SP, Silva MT. Ecstasy (MDMA): effects and patterns of use reported by users in Sao Paulo. Rev Bras.Psiquiatr. 2003;25:11–17. doi: 10.1590/s1516-44462003000100004. [DOI] [PubMed] [Google Scholar]
- de Win MM, Jager G, Vervaeke HK, Schilt T, Reneman L, Booij J, Verhulst FC, den Heeten GJ, Ramsey NF, Korf DJ, van den BW. The Netherlands XTC Toxicity (NeXT) study: objectives and methods of a study investigating causality, course, and clinical relevance. Int.J Methods Psychiatr.Res. 2005;14:167–185. doi: 10.1002/mpr.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Win MM, Reneman L, Reitsma JB, den Heeten GJ, Booij J, van den BW. Mood disorders and serotonin transporter density in ecstasy users--the influence of long-term abstention, dose, and gender. Psychopharmacology (Berl) 2004;173:376–382. doi: 10.1007/s00213-003-1723-4. [DOI] [PubMed] [Google Scholar]
- Easton N, Marsden CA. Ecstasy: are animal data consistent between species and can they translate to humans? J Psychopharmacol. 2006;20:194–210. doi: 10.1177/0269881106061153. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Fox HC, Parrott AC, Turner JJ. Ecstasy use: cognitive deficits related to dosage rather than self-reported problematic use of the drug. J Psychopharmacol. 2001;15:273–281. doi: 10.1177/026988110101500406. [DOI] [PubMed] [Google Scholar]
- Friedman L, Kenny JT, Wise AL, Wu D, Stuve TA, Miller DA, Jesberger JA, Lewin JS. Brain activation during silent word generation evaluated with functional MRI. Brain Lang. 1998;64:231–256. doi: 10.1006/brln.1998.1953. [DOI] [PubMed] [Google Scholar]
- Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2006;139:251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gibb JW, Johnson M, Stone D, Hanson GR. MDMA: historical perspectives. Ann.N.Y.Acad.Sci. 1990;600:601–611. doi: 10.1111/j.1749-6632.1990.tb16913.x. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. The confounding problem of polydrug use in recreational ecstasy/MDMA users: a brief overview. J Psychopharmacol. 2006;20:188–193. doi: 10.1177/0269881106059939. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Fischermann T, Rezk M, Thimm B, Hensen G, Daumann J. Memory performance in polyvalent MDMA (ecstasy) users who continue or discontinue MDMA use. Drug Alcohol Depend. 2005;78:317–323. doi: 10.1016/j.drugalcdep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Herholz K, Ehlen P, Kessler J, Strotmann T, Kalbe E, Markowitsch HJ. Learning face-name associations and the effect of age and performance: a PET activation study. Neuropsychologia. 2001;39:643–650. doi: 10.1016/s0028-3932(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Herning RI, Better WE, Tate K, Cadet JL. Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology. 2005;64:488–493. doi: 10.1212/01.WNL.0000150882.69371.DD. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Preliminary evidence of hippocampal dysfunction in adolescent MDMA (“ecstasy”) users: possible relationship to neurotoxic effects. Psychopharmacology (Berl) 2004;173:383–390. doi: 10.1007/s00213-003-1679-4. [DOI] [PubMed] [Google Scholar]
- Jager G, de Win MM, van d T,I, Schilt T, Kahn RS, van den BW, van Ree JM, Ramsey NF. Assessment of cognitive brain function in ecstasy users and contributions of other drugs of abuse: results from an FMRI study. Neuropsychopharmacology. 2008;33:247–258. doi: 10.1038/sj.npp.1301415. [DOI] [PubMed] [Google Scholar]
- Jager G, de Win MM, Vervaeke HK, Schilt T, Kahn RS, van den BW, van Ree JM, Ramsey NF. Incidental use of ecstasy: no evidence for harmful effects on cognitive brain function in a prospective fMRI study. Psychopharmacology (Berl) 2007;193:403–414. doi: 10.1007/s00213-007-0792-1. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JG. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2007. National Institute on Drug Abuse; Bethesda, MD: 2008. (NIH Publication No. 08-6418) [Google Scholar]
- Kalechstein AD, De La GR, Mahoney JJ, III, Fantegrossi WE, Newton TF. MDMA use and neurocognition: a meta-analytic review. Psychopharmacology (Berl) 2007;189:531–537. doi: 10.1007/s00213-006-0601-2. [DOI] [PubMed] [Google Scholar]
- Kish SJ. How strong is the evidence that brain serotonin neurons are damaged in human users of ecstasy? Pharmacol.Biochem.Behav. 2002;71:845–855. doi: 10.1016/s0091-3057(01)00708-0. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum.Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws KR, Kokkalis J. Ecstasy (MDMA) and memory function: a meta-analytic update. Hum.Psychopharmacol. 2007;22:381–388. doi: 10.1002/hup.857. [DOI] [PubMed] [Google Scholar]
- Lee AC, Robbins TW, Graham KS, Owen AM. “Pray or Prey” dissociation of semantic memory retrieval from episodic memory processes using positron emission tomography and a novel homophone task. Neuroimage. 2002;16:724–735. doi: 10.1006/nimg.2002.1101. [DOI] [PubMed] [Google Scholar]
- Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13:257–267. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Magn Reson.Imaging. 2004;22:1517–1531. doi: 10.1016/j.mri.2004.10.018. [DOI] [PubMed] [Google Scholar]
- McCann UD, Eligulashvili V, Ricaurte GA. (+/−)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: clinical studies. Neuropsychobiology. 2000;42:11–16. doi: 10.1159/000026665. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/−)3,4-methylenedioxymethamphetamine (“ecstasy”) users: relationship to cognitive performance. Psychopharmacology (Berl) 2008;200:439–450. doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittenberg W, Motta S. Effects of chronic cocaine abuse on memory and learning. Arch.Clin Neuropsychol. 1993;8:477–483. [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Dougherty DM, Narayana PA, Kramer LA, Renshaw PF. Functional MRI study of working memory in MDMA users. Psychopharmacology (Berl) 2004;177:185–194. doi: 10.1007/s00213-004-1908-5. [DOI] [PubMed] [Google Scholar]
- Morgan MJ. Ecstasy (MDMA): a review of its possible persistent psychological effects. Psychopharmacology (Berl) 2000;152:230–248. doi: 10.1007/s002130000545. [DOI] [PubMed] [Google Scholar]
- Obrocki J, Buchert R, Vaterlein O, Thomasius R, Beyer W, Schiemann T. Ecstasy--long-term effects on the human central nervous system revealed by positron emission tomography. Br.J Psychiatry. 1999;175:186–8. doi: 10.1192/bjp.175.2.186. 186-188. [DOI] [PubMed] [Google Scholar]
- Obrocki J, Schmoldt A, Buchert R, Andresen B, Petersen K, Thomasius R. Specific neurotoxicity of chronic use of ecstasy. Toxicol.Lett. 2002;127:285–297. doi: 10.1016/s0378-4274(01)00511-2. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Morgan PT, Malison RT, Hart CL, Edgar C, Walker M, Stickgold R. Cocaine users differ from normals on cognitive tasks which show poorer performance during drug abstinence. Am J Drug Alcohol Abuse. 2008;34:109–121. doi: 10.1080/00952990701764821. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA in humans: factors which affect the neuropsychobiological profiles of recreational ecstasy users, the integrative role of bioenergetic stress. J Psychopharmacol. 2006;20:147–163. doi: 10.1177/0269881106063268. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch.Gen.Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. The role of fMRI in Cognitive Neuroscience: where do we stand? Curr Opin Neurobiol. 2008 doi: 10.1016/j.conb.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch.Gen.Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc.Natl.Acad.Sci.U.S.A. 2008;105:6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneman L, Booij J, de BK, Reitsma JB, de Wolff FA, Gunning WB, den Heeten GJ, van den BW. Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet. 2001a;358:1864–1869. doi: 10.1016/S0140-6736(01)06888-X. [DOI] [PubMed] [Google Scholar]
- Reneman L, Booij J, Schmand B, van den BW, Gunning B. Memory disturbances in “Ecstasy” users are correlated with an altered brain serotonin neurotransmission. Psychopharmacology (Berl) 2000a;148:322–324. doi: 10.1007/s002130050057. [DOI] [PubMed] [Google Scholar]
- Reneman L, Endert E, de Bruin K, Lavalaye J, Feenstra MG, de Wolff FA, Booij J. The acute and chronic effects of MDMA (“ecstasy”) on cortical 5-HT2A receptors in rat and human brain. Neuropsychopharmacology. 2002;26:387–396. doi: 10.1016/S0893-133X(01)00366-9. [DOI] [PubMed] [Google Scholar]
- Reneman L, Habraken JB, Majoie CB, Booij J, den Heeten GJ. MDMA (“Ecstasy”) and its association with cerebrovascular accidents: preliminary findings. AJNR Am J Neuroradiol. 2000b;21:1001–1007. [PMC free article] [PubMed] [Google Scholar]
- Reneman L, Lavalaye J, Schmand B, de Wolff FA, van den BW, den Heeten GJ, Booij J. Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”): preliminary findings. Arch.Gen.Psychiatry. 2001b;58:901–906. doi: 10.1001/archpsyc.58.10.901. [DOI] [PubMed] [Google Scholar]
- Reneman L, Majoie CB, Habraken JB, den Heeten GJ. Effects of ecstasy (MDMA) on the brain in abstinent users: initial observations with diffusion and perfusion MR imaging. Radiology. 2001c;220:611–617. doi: 10.1148/radiol.2202001602. [DOI] [PubMed] [Google Scholar]
- Reneman L, Majoie CB, Schmand B, van den BW, den Heeten GJ. Prefrontal N-acetylaspartate is strongly associated with memory performance in (abstinent) ecstasy users: preliminary report. Biol.Psychiatry. 2001d;50:550–554. doi: 10.1016/s0006-3223(01)01177-5. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Yuan J, McCann UD. (+/−)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology. 2000;42:5–10. doi: 10.1159/000026664. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Simonotto E, Spencer EP, Ebmeier KP. The effects of escitalopram on working memory and brain activity in healthy adults during performance of the n-back task. Psychopharmacology (Berl) 2006;185:339–347. doi: 10.1007/s00213-006-0334-2. [DOI] [PubMed] [Google Scholar]
- Schilt T, de Win MM, Koeter M, Jager G, Korf DJ, van den BW, Schmand B. Cognition in novice ecstasy users with minimal exposure to other drugs: a prospective cohort study. Arch.Gen.Psychiatry. 2007;64:728–736. doi: 10.1001/archpsyc.64.6.728. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Wingen M, Ramaekers JG, Evers EA, Riedel WJ. Serotonin and human cognitive performance. Curr.Pharm.Des. 2006;12:2473–2486. doi: 10.2174/138161206777698909. [DOI] [PubMed] [Google Scholar]
- Scholey AB, Parrott AC, Buchanan T, Heffernan TM, Ling J, Rodgers J. Increased intensity of Ecstasy and polydrug usage in the more experienced recreational Ecstasy/MDMA users: a WWW study. Addict.Behav. 2004;29:743–752. doi: 10.1016/j.addbeh.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC. Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy’) users. Br.J Psychiatry. 1999;175:63–69. doi: 10.1192/bjp.175.1.63. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Pope HG, Jr., Silveri MM, Simpson NS, Gruber SA, Yurgelun-Todd DA. Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. Eur.Neuropsychopharmacol. 2008;18:612–619. doi: 10.1016/j.euroneuro.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, Rayport M. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. Georg Thieme Verlag; 1988. [Google Scholar]
- Thomasius R, Petersen K, Buchert R, Andresen B, Zapletalova P, Wartberg L, Nebeling B, Schmoldt A. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users. Psychopharmacology (Berl) 2003;167:85–96. doi: 10.1007/s00213-002-1383-9. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, ia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crimes . 2008 World Drug Report. United Nations Publications; Vienna: 2008. [Google Scholar]
- van Reekum R, Streiner DL, Conn DK. Applying Bradford Hill’s criteria for causation to neuropsychiatry: challenges and opportunities. J Neuropsychiatry Clin Neurosci. 2001;13:318–325. doi: 10.1176/jnp.13.3.318. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Smith EE, Nichols TE. Toward a taxonomy of attention shifting: individual differences in fMRI during multiple shift types. Cogn Affect.Behav.Neurosci. 2005;5:127–143. doi: 10.3758/cabn.5.2.127. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Morales M, Rothman RB. (+/−)-3,4-Methylenedioxymethamphetamine administration to rats does not decrease levels of the serotonin transporter protein or alter its distribution between endosomes and the plasma membrane. J Pharmacol Exp.Ther. 2005;314:1002–1012. doi: 10.1124/jpet.105.088476. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Rothman RB. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse. 2004;53:240–248. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]
- Wish ED, Fitzelle DB, O’Grady KE, Hsu MH, Arria AM. Evidence for significant polydrug use among ecstasy-using college students. J Am.Coll.Health. 2006;55:99–104. doi: 10.3200/JACH.55.2.99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Arch.Gen.Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Young DA, Radkhoshnoud NF. Attentional processes in abstinent methylenedioxymethamphetamine (ecstasy) users. Appl Neuropsychol. 2002;9:84–91. doi: 10.1207/S15324826AN0902_3. [DOI] [PubMed] [Google Scholar]