Abstract
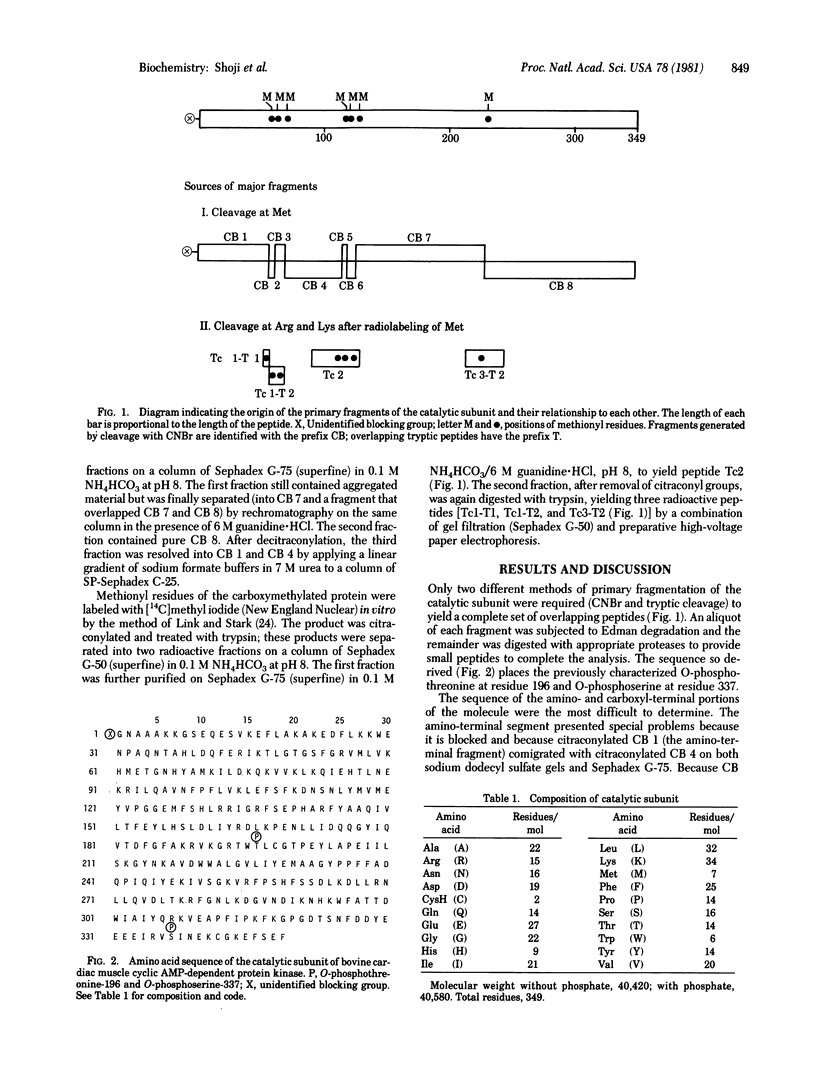
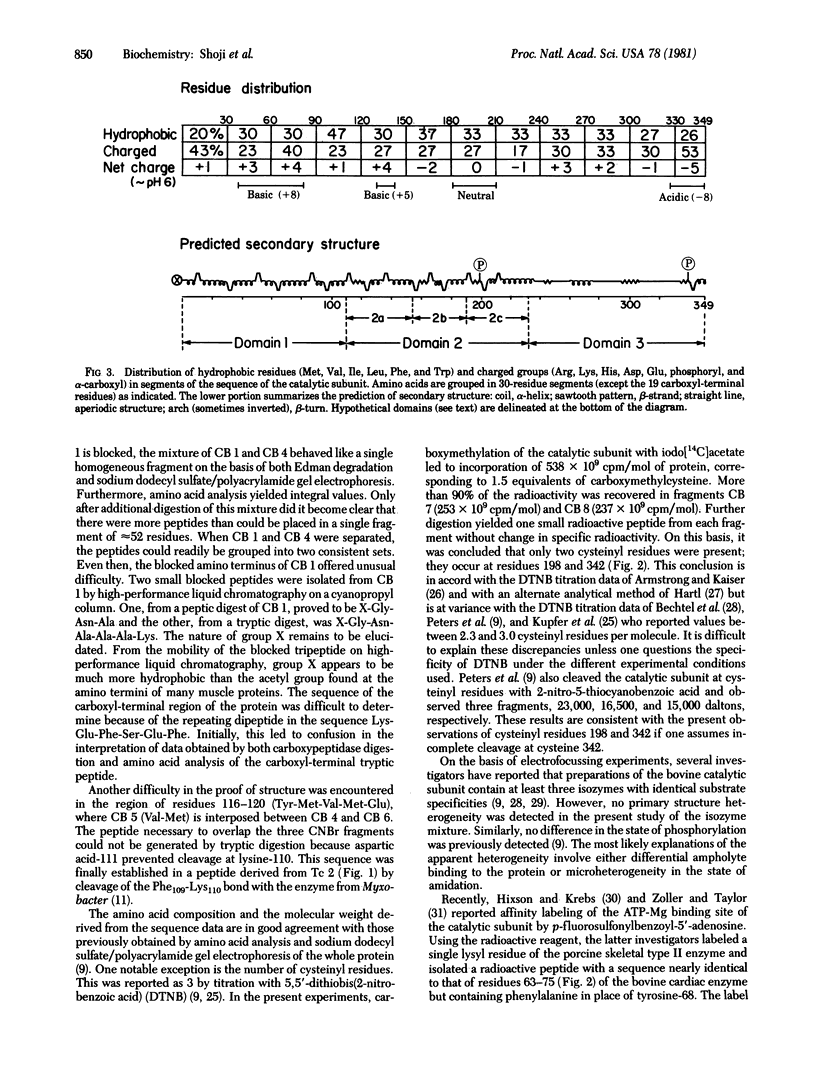
The complete amino acid sequence of the 349-residue catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle is presented. The sequence of the subunit (Mr 40,580 including phosphate groups at threonine-196 and serine-337) was derived largely by automated Edman degradation of nine fragments generated from the carboxymethylated protein by cleavage of methionyl bonds with cyanogen bromide. These fragments were aligned along the polypeptide chain by analysis of methionine-containing tryptic peptides isolated from protein radiolabeled in vitro by [14C]methyl exchange at methionyl residues. The molecule contains only two cysteinyl residues, at positions 198 and 342. It is relatively polar, containing clusters of cationic residues toward the amino terminus and anionic residues towards the carboxyl terminus. Predictions of secondary structure suggest the presence of three major domains with approximately half of the residues occurring in alpha-helices and 12% in beta-strands.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. N., Kaiser E. T. Sulfhydryl group reactivity of adenosine 3',5'-monophosphate dependent protein kinase from bovine heart: a probe of holoenzyme structure. Biochemistry. 1978 Jul 11;17(14):2840–2845. doi: 10.1021/bi00607a022. [DOI] [PubMed] [Google Scholar]
- Bechtel P. J., Beavo J. A., Krebs E. G. Purification and characterization of catalytic subunit of skeletal muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Apr 25;252(8):2691–2697. [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Daile P., Carnegie P. R., Young J. D. Synthetic substrate for cyclic AMP-dependent protein kinase. Nature. 1975 Oct 2;257(5525):416–418. doi: 10.1038/257416a0. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. Evaluation of several amino group reversible blocking reagents. Biochemistry. 1970 Dec 8;9(25):4939–4944. doi: 10.1021/bi00827a016. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Hixson C. S., Krebs E. G. Affinity labeling of catalytic subunit of bovine heart muscle cyclic AMP-dependent protein kinase by 5'-p-fluorosulfonylbenzoyladenosine. J Biol Chem. 1979 Aug 25;254(16):7509–7514. [PubMed] [Google Scholar]
- Hjelmquist G., Andersson J., Edlund B., Engstroöm L. Amino acid sequence of a (32P) phosphopeptide from pig liver pyruvate kinase phosphorylated by cyclic 3',5'-AMP-stimulated protein kinase and gamma-(32P)ATP. Biochem Biophys Res Commun. 1974 Nov 27;61(2):559–563. doi: 10.1016/0006-291x(74)90993-0. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Beavo J. A., Bechtel P. J., Krebs E. G. Comparison of adenosine 3':5'-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975 Oct 10;250(19):7795–7801. [PubMed] [Google Scholar]
- Horn M. J., Laursen R. A. Solid-phase edman degradation: attachment of carboxyl-terminal homoserine peptides to an insoluble resin. FEBS Lett. 1973 Nov 1;36(3):285–288. doi: 10.1016/0014-5793(73)80392-8. [DOI] [PubMed] [Google Scholar]
- Huang T. S., Bylund D. B., Stull J. T., Krebs E. G. The amino acid sequences of the phosphorylated sites in troponin-I from rabbit skeletal muscle. FEBS Lett. 1974 Jun 15;42(3):249–252. doi: 10.1016/0014-5793(74)80738-6. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Bylund D. B., Huang T. S., Krebs E. G. Substrate specificity of the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3448–3452. doi: 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Kupfer A., Gani V., Jiménez J. S., Shaltiel S. Affinity labeling of the catalytic subunit of cyclic AMP-dependent protein kinase by N alpha-tosyl-L-lysine chloromethyl ketone. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3073–3077. doi: 10.1073/pnas.76.7.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan T. A. Cyclic AMP and histone phosphorylation. Ann N Y Acad Sci. 1971 Dec 30;185:166–180. doi: 10.1111/j.1749-6632.1971.tb45246.x. [DOI] [PubMed] [Google Scholar]
- Laursen R. A., Horn M. J., Bonner A. G. Solid-phase Edman degradation. The use of p-phenyl diisothiocyanate to attach lysine- and arginine-containing peptides to insoluble resins. FEBS Lett. 1972 Mar;21(1):67–70. doi: 10.1016/0014-5793(72)80165-0. [DOI] [PubMed] [Google Scholar]
- Laursen R. A. Solid-phase Edman degradation. An automatic peptide sequencer. Eur J Biochem. 1971 May 11;20(1):89–102. doi: 10.1111/j.1432-1033.1971.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Link T. P., Stark G. R. S-methylmethionine-29 ribonuclease A. I. Preparation and proof of structure. J Biol Chem. 1968 Mar 25;243(6):1082–1088. [PubMed] [Google Scholar]
- Peters K. A., Demaille J. G., Fischer E. H. Adenosine 3':5'-monophosphate dependent protein kinase from bovine heart. Characterization of the catalytic subunit. Biochemistry. 1977 Dec 27;16(26):5691–5697. doi: 10.1021/bi00645a007. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Rosen O. M., Rangel-Aldao R., Erlichman J. Soluble cyclic AMP-dependent protein kinases: review of the enzyme isolated from bovine cardiac muscle. Curr Top Cell Regul. 1977;12:39–74. [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Molecular forms and subunit composition of a cyclic adenosine 3',5'-monophosphate-dependent protein kinase purified from bovine heart muscle. J Biol Chem. 1972 Jan 10;247(1):36–44. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Shoji S., Titani K., Demaille J. G., Fischer E. H. Sequence of two phosphorylated sites in the catalytic subunit of bovine cardiac muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1979 Jul 25;254(14):6211–6214. [PubMed] [Google Scholar]
- Sugden P. H., Holladay L. A., Reimann E. M., Corbin J. D. Purification and characterization of the catalytic subunit of adenosine 3':5'-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J. 1976 Nov;159(2):409–422. doi: 10.1042/bj1590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Wingard M., Matsueda G., Wolfe R. S. Myxobacter AL-1 protease II: specific peptide bond cleavage on the amino side of lysine. J Bacteriol. 1972 Nov;112(2):940–949. doi: 10.1128/jb.112.2.940-949.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman S. J., Cohen P. The specificity of adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Biochem Soc Trans. 1976;4(6):1027–1030. doi: 10.1042/bst0041027. [DOI] [PubMed] [Google Scholar]
- Zetterqvist O., Ragnarsson U., Humble E., Berglund L., Engström L. The minimum substrate of cyclic AMP-stimulated protein kinase, as studied by synthetic peptides representing the phosphorylatable site of pyruvate kinase (type L) of rat liver. Biochem Biophys Res Commun. 1976 Jun 7;70(3):696–703. doi: 10.1016/0006-291x(76)90648-3. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Taylor S. S. Affinity labeling of the nucleotide binding site of the catalytic subunit of cAMP-dependent protein kinase using p-fluorosulfonyl-[14C]benzoyl 5'-adenosine. Identification of a modified lysine residue. J Biol Chem. 1979 Sep 10;254(17):8363–8368. [PubMed] [Google Scholar]
- de Haën C., Swanson E., Teller D. C. The evolutionary origin of proinsulin. Amino acid sequence homology with the trypsin-related serine proteases detected and evaluated by new statistical methods. J Mol Biol. 1976 Sep 25;106(3):639–661. doi: 10.1016/0022-2836(76)90256-4. [DOI] [PubMed] [Google Scholar]


