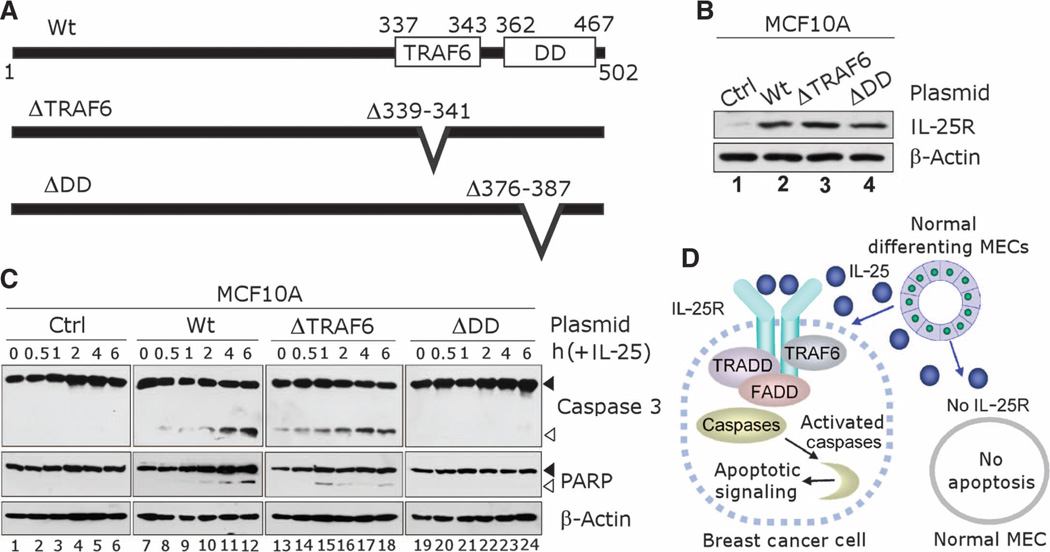
Fig. 7.
Death domain (DD)–like region of IL-25R renders cells sensitive to apoptotic signaling after treatment with IL-25. (A) Schematics for IL-25R protein expressed in mutation analyses. Wt, wild-type full-length IL-25R protein; ΔTRAF6, mutated IL-25R with a deletion in TRAF6 binding domain (amino acids Δ339–341); ΔDD, mutated IL-25R with a deletion in the DD-like region (amino acids Δ376–387). (B) Western blot shows that increased IL-25R amounts (Wt, ΔTRAF6, or ΔDD) after IL-25R were ectopically expressed in MCF10A cells compared to the endogenous IL-25R amounts in parental cells (Ctrl). β-Actin served as an internal control. (C) DD-like domain of IL-25R is essential for apoptotic signaling mediated by IL-25. Western blot analysis was used to detect cleavage of caspase 3 and PARP in MCF10A cells that expressed IL-25R as in (B) after treatment with IL-25 (500 ng/ml, ~25 nM) for varying time periods. β-Actin served as an internal control. Black arrowheads, uncleaved protein; white arrowheads, cleaved protein. (D) Schematic for the cytotoxic activity of IL-25 specific to breast cancer cells that express IL-25R. Nonmalignant MECs do not express IL-25R and are resistant to apoptosis induced by IL-25. Breast cancer cells that express IL-25R are susceptible to IL-25–induced apoptosis.