Abstract
Strychnine-sensitive glycine receptors (GlyR) play a major role in the excitability of CNS neurons and are also a major target of many drugs including some general anesthetics and ethanol. The prefrontal cortex (PFC) is an important substrate responsible for cognitive function and for sedation, as well as hypnosis (unconsciousness) which is induced by general anesthetics and ethanol. However, the functions and the physiological and pharmacological properties of GlyRs in mature PFC neurons have not been well studied. In this study, whole-cell currents induced by glycine (IGly) were recorded from freshly isolated PFC neurons of Sprague-Dawley rats aged 5 to 39 postnatal days (neonatal, P5–12; weanling, P17–21 and peri-adolescent, P30–39). We found that most of the neurons examined were responsive to glycine and the response was concentration dependent. With the increase of age, the sensitivity to glycine was significantly decreased and the sensitivity to picrotoxin was significantly increased. Conversely, the changes in sensitivity to strychnine were not significant. Interestingly, IGly of all age groups was suppressed (to different scope) by low concentrations of picrotoxin (≤ 30 µM), which selectively blocked α homomeric GlyRs. Conversely, about 20–65% of IGly remained in the presence of 300 µM picrotoxin, suggesting the picrotoxin-resistant subtype the αβ heteromeric GlyR, was also present. These data provide the first evidence that there are at least two subtypes of functional GlyRs in the PFC neurons of young rats, and their physiological and pharmacological properties change substantially during maturation.
Keywords: whole cell patch-clamp, prefrontal cortex, glycine receptor, strychnine, picrotoxin
1. Introduction
Strychnine-sensitive glycine receptors (GlyR) mediate inhibitory neurotransmission in the spinal cord and other regions of the CNS (Aprison and Werman, 1965; Betz, 1991), and are a major target of many drugs including some general anesthetics (Nguyen et al., 2009) and ethanol (Aguayo and Pancetti, 1994; Ye et al., 2001; Ye et al., 2009). GlyR contains four α-subunits (1–4) and one β-subunit. Previous studies have indicated that in naïve neurons, functional GlyRs are comprised of α-homomers and α-β heteromers with a subunit stoichiometry of 2α3β (Grenningloh et al., 1987; Grenningloh et al., 1990; Grudzinska et al., 2005; Lynch, 2009) and that the subunit composition and their assembly change with development (Lynch, 2009).
The prefrontal cortex (PFC) is one of the last territories of the neocortex in evolution as well as ontogeny (Fuster, 2001). It has long been implicated to play an important role in cognitive control via the ability to orchestrate thought and action in accordance with internal goals (Fuster, 2001). The cortical networks are the major neuroanatomical substrate responsible for the sedation and hypnosis (unconsciousness), among other effects induced by ethanol (Ye et al., 2009) and some general anesthetics such as propofol. Although previous studies using in situ hybridization and quantitative PCR (qPCR) have indicated that α and β subunits of the GlyR are expressed in the rats’ cerebral cortex during early development and adult stage (Jonsson et al., 2009; Malosio et al., 1991), little is known regarding the physiological and pharmacological properties including the developmental changes of these receptors in the PFC. Although functional GlyRs have been demonstrated in embryonic and P0–P4 cortical neurons of rats (Flint et al., 1998), they have not been demonstrated in more mature animals. Furthermore, since recent clinical research has been testing inhibitors of the glycine transporter 1 as therapeutics for the negative symptoms of schizophrenia; knowledge on GlyRs in PFC is important and timely, since these receptors may also be activated with increased ambient glycine levels in the PFC. To this end, in the present study, we recorded whole-cell glycine currents from neurons freshly isolated from PFC of Sprague-Dawley rats of postnatal days 5–39 (P5–39).
2. Results
Neurons were grouped according to the postnatal age of the animal from which they were obtained (day of birth = P0): neonatal, P5–12; weanling, P17–21; and peri-adolescent, P30–39 (McCutcheon and Marinelli, 2009). These groups were selected in light of the developmental changes associated with GlyR structure and the physiological and pharmacological properties in rat neurons.
2.1. IGly in the PFC neurons
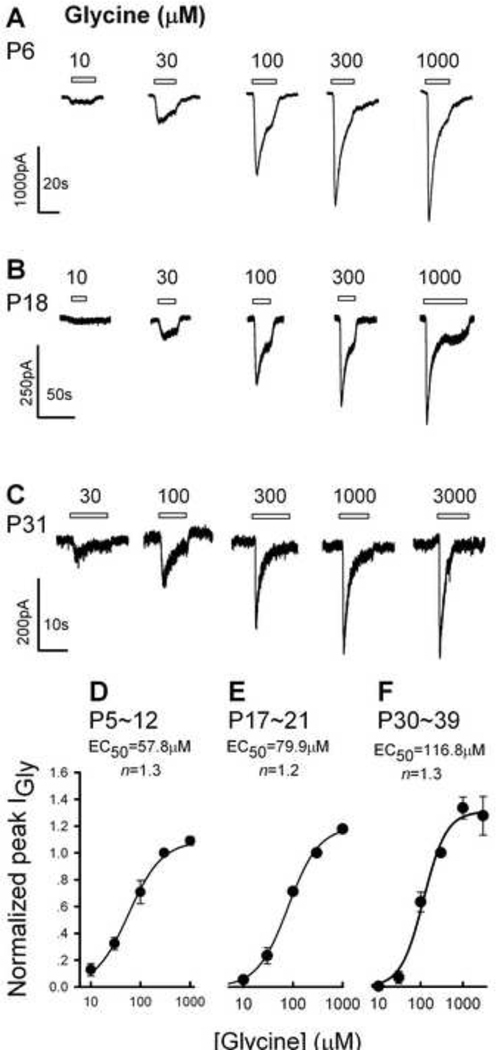
The application of glycine induced an inward current in the majority of neurons examined at a holding potential of −50 mV: for P5–12 rats, 94% (29/31) of the PFC neurons examined produced IGly in response to glycine, 83% (40/48) and 53% (30/56) for P17–21 and P30–39 rats, respectively (Table 1). IGly was characterized by a fast onset followed by a slower decay in the continuous presence of the agonist (Fig.1A, 1B, 1C). IGly increased in amplitude sigmoidally with the concentration of the agonist (Fig. 1D, 1E, 1F). The EC50s and Hill coefficients were 57.8 ± 3.7 µM (mean ± SEM) and 1.3 for PFC neurons from P5–12 rats (Fig. 1D); 79.9 ± 7.2 µM and 1.4 for P17–21 rats (Fig. 1C); and 116.8 ± 17.9 µM and 1.3 for P30–39 rats (Fig. 1F), respectively. One way ANOVA revealed significant difference between P5–12 and P31–39 groups (F2,12 = 6.9, P = 0.008), and between P17–21 and P31–39 groups (P = 0.04), the difference between P5–12 and P17–21 (P = 0.19), was not significant. Thus, the EC50 of IGly increased with the age of the rats, indicating that physiological properties of GlyRs in the PFC changed during early life.
Table 1.
EC50 or IC50 (mean ±SEM, µM) and Hill coefficient (n) of three age groups
| P5–12 | P17–21 | P30–39 | |
|---|---|---|---|
| Glycine response | 94% (29/31) | 83%(40/48) | 53%(30/56) |
| Glycine EC50 | 57.8±3.7 (n=1.3) | 79.9±7.2(n=1.4) | 116.8±17.9(n=1.3) |
| Strychnine IC50 | 0.16±0.02(n=1.0) | 0.25±0.01(n=1.4) | 0.13±0.09(n=1.3) |
| Picrotoxin IC50 | 690.1±91.2(n=0.7) | 250.0±41.7(n=0.8) | 67.1±4.8(n=0.9) |
Fig. 1.
IGly of PFC neurons. Typical IGly traces of PFC neurons from an 6-day-old rat (A), a 18-day-old rat (B) and a 31-day-old rat (C). Holding potential VH was −50 mV in all cells. Concentration-response curves of IGly for the 5–12day-old (D), 17–21-day-old (E) and 30–39-day-old (F) neurons. All points were normalized to the peak response elicited by 300 µM glycine. Each point is the mean of three to seven cells and the vertical bars show ± S.E.M. The glycine concentration producing a half-maximal response (EC50) and the Hill coefficient (n) were estimated using the Michaelis-Menten equation: I= (ImaxCn)/(Cn + (EC50)n), where I is the observed IGly, Imax the maximum current, and C the glycine concentration. The EC50 value and the Hill coefficient were 57.8 µM and 1.3, 79.9 µM and 1.2 for the 17–21-day-old, and 116.8 µM and 1.3 for the 30–39-day-old neurons.
2.2. Effects of strychnine on IGly
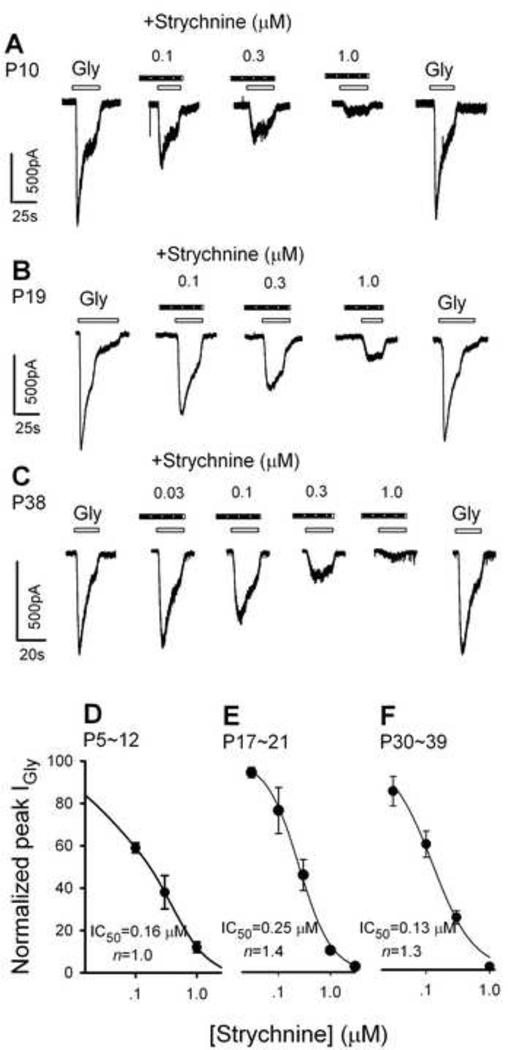
The plant alkaloid, strychnine, is a selective antagonist for GlyRs (Young and Snyder, 1973; Young and Snyder, 1974). The α subunits of GlyRs carry the binding site for strychnine. To characterize the pharmacological properties of the GlyRs of PFC neurons, we tested the effects of strychnine on IGly recorded from all three age groups. As illustrated in Fig. 2A, 2B and 2C, glycine (500 µM) was first applied alone to obtain an IGly baseline. Strychnine (STR, 0.03–1.0 µM) was then applied for 10–20 seconds, followed by a solution containing the mixture of strychnine and glycine. After washout of the mixture, glycine alone was applied again. The peak amplitudes of the IGly obtained under these different conditions were measured. The percentage of inhibition of IGly by strychnine was calculated by the formula (B/((A+C)/2) × 100, where (A) is the amplitude of IGly during baseline conditions, (B) during strychnine application, and (C) after washout of strychnine.
Fig. 2.
Strychnine suppression of IGly. Typical IGly traces of PFC neurons from a 10-day-old rat (A), a 19-day-old rat (B) and a 38-day-old rat (C) in response to 500 µM glycine in the absence and presence of strychnine. Strychnine was applied a few seconds before the application of glycine. (D, E, F) concentration-response relationships of strychnine blockage of IGly. After normalizing the peak IGly in the presence of strychnine to the control value, the mean ± S.E.M. was calculated and plotted as a function of strychnine concentrations. Each point represents the mean of three to four cells. VH is −50 mV. For estimation of the dissociation constant (Kd) and the Hill coefficient (n) of the concentration-response curve, the following form of the Logistic equation was fit to the data, I/IGly=1/ (1+(C+ (Kd) n)). Where I is the current with strychnine, IGly is the control current, C is the concentration of strychnine. The IC50 and the Hill coefficient were calculated to be 0.16 µM and 1.0 for the 5–12-day-old, 0.25 µM and 1.4 for the 17–21-day-old, and 0.13 µM and 1.3 for the 30–39-day-old neurons, respectively.
The data showed that strychnine concentration dependently reduced IGly in neurons of all age groups of rats (Fig. 2A, 2B, 2C). Figures 2D, 2E and 2F showed plots of the relationship between the peak of IGly (normalized to the control value) and the strychnine concentrations for P5–12, P17–21 and P30–39 neurons, respectively. Inhibition curves fit to these data yielded strychnine IC50 of 0.16 ± 0.02 µM (mean ± SEM) for P5–12 neurons, 0.25 ± 0.01 µM for P17–21 neurons and 0.13 ± 0.09 µM for P30–39 neurons. One way ANOVA revealed no significant difference among these three groups (F2,10 = 1.14, P = 0.36).
2.3. Effects of picrotoxin on IGly
The GABAA antagonist picrotoxin is a useful tool in differentiating between homomeric and heteromeric GlyRs. Previous studies have shown that low concentrations of picrotoxin suppresses the function of α homomeric receptors, but affects the function of α+β heteromeric receptors less (Chattipakorn and McMahon, 2002; Pribilla et al., 1992). In order to obtain further information for the subunit structure of native GyRs in the PFC, we tested the effect of picrotoxin on IGly of neurons of all three age groups.
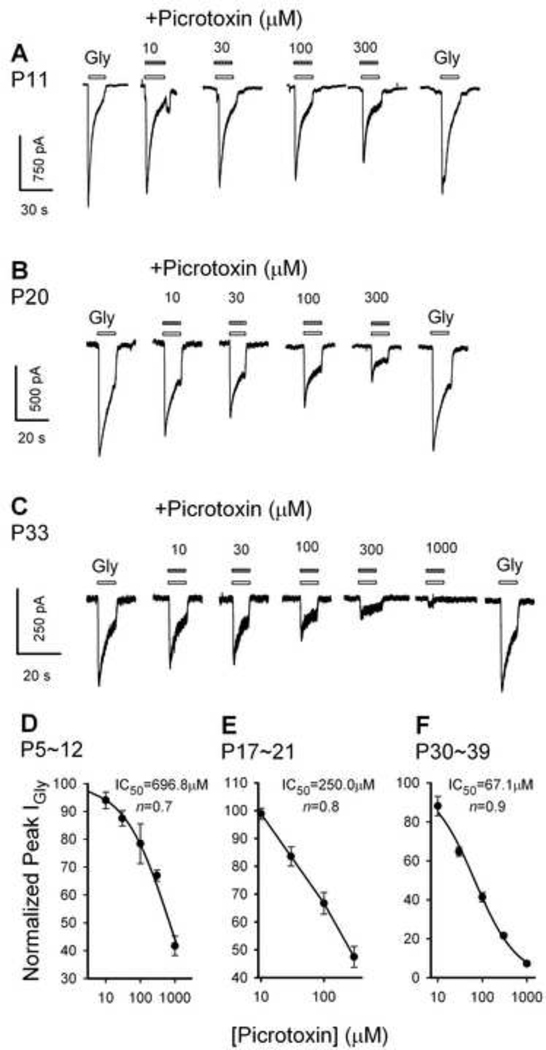
Picrotoxin concentration dependently reduced glycine IGly in neurons of all age groups (Fig. 3A, 3B and 3C). The relationship between the peak amplitude of IGly (normalized to the peak amplitude of the control IGly induced by 500 µM glycine) and the concentrations of picrotoxin for P5–12, P17–21 and P30–39 neurons are illustrated in Fig. 3D, 3E and 3F, respectively. Inhibition curves were fit to these data and the results yielded a picrotoxin IC50 of 696.8 ± 97.0 µM (mean ± SEM) for P5–12 neurons; 250.0 ± 41.7 µM for P17–21 neurons; and 67.1 ± 4.8 µM for P30–39 neurons. One way ANOVA revealed significant difference among groups (F2, 8 = 39.48, P < 0.001). Post hoc analysis revealed: between P5–12 and P31–39 groups (P < 0.001), between P5–12 and P17–21 (P < 0.001), and between P17–21 and P31–39 (P = 0.025). Thus, the sensitivity of GlyRs to picrotoxin significantly increased with age.
Fig. 3.
Picrotoxin suppression of IGly. Typical IGly traces of PFC neurons from a 11-day-old rat (A), a 20-day-old rat (B) and a 33-day-old rat (C) in response to 500 µM glycine in the absence and presence of picrotoxin. VH was −50 mV. Picrotoxin was co-applied with glycine. (D, E, F) concentration-response relation of picrotoxin blockage of IGly. After normalizing the peak IGly in the presence of picrotoxin to the control value, the mean ±S.E.M. was calculated and plotted as a function of picrotoxin concentrations. Each point represents the mean of three to six cells. The dissociation constant (Kd) and the Hill coefficient (n) of the concentration-response curve were estimated using the Logistic equation described above in the legend of Fig. 2, where I is the current with picrotoxin, IGly is the control current, C is the concentration of picrotoxin. The IC50 and the Hill coefficient were 690.1 µM and 0.7 for the 5–12-day-old, 250.0 µM and 0.8 for the 17–21-day-old, and 67.1 µM and 0.9 for the 30–39-day-old neurons, respectively.
3. Discussion
GlyRs are classically known for mediating inhibitory synaptic transmission in the CNS. In the beginning, on the basis of autoradiography with the GlyR antagonist strychnine, GlyRs were thought to be mainly concentrated in spinal cord, brain stem and other areas of the lower neuraxis and were absent from the cerebral cortex and other rostral regions of the CNS (Frostholm and Rotter, 1985). Using in situ hybridization technique, α2 and β subunits of the GlyR were confirmed in cerebral cortex/PFC during early development (Malosio et al., 1991). While the β subunit persists into adulthood, a2 subunit declines sharply following the first postnatal week and remains only at a low level in the adult neocortex (Jonsson et al., 2009; Malosio et al., 1991; Sato et al., 1992). Although a previous electrophysiology study showed that functional GlyRs exist in the PFC neurons of embryonic and early postnatal (P0–P4) (Flint et al., 1998), functional GlyRs in the PFC of older animals have not been demonstrated. In the present study, by using patch clamp technique and pharmacological approaches, we have, for the first time, provided strong evidence that functional GlyRs exist in PFC neurons of rats aged 5–39 postnatal days.
Developmental change is one of the important features of GlyRs. Several previous studies have shown that GlyR mRNAs in the fetal rat are predominantly α2 homomers (Becker et al., 1988; Lynch, 2009; Malosio et al., 1991; Watanabe and Akagi, 1995). In the spinal cord and lower brain stem, while α2 expression dramatically subsides between birth and postnatal week three, expression of α1 and β subunits increases over the same period. Thus, it has been proposed that in the maturation of the CNS of the rat, there is a developmental switch from α2 homomers to α1β heteromers (Lynch, 2009). This notion is also supported by the electrophysiological evidence from several brain areas including the medial nucleus of the trapezoid body(Kungel and Friauf, 1997), the VTA (Ye, 2000), and the rat substantia nigra (Mangin et al., 2002).
In the current study, we showed that the glycine sensitivity of PFC neurons decreased with age, which was the opposite to that in the acutely isolated VTA neurons and cultured spinal cord neurons where the agonist sensitivity increased with time (Tapia and Aguayo, 1998; Ye, 2000). However, the sensitivity to glycine may not provide information on the subunit composition of the receptors, since there is no evidence to date for subunit-specific GlyR agonists. All homomeric and heteromeric GlyR subtypes exhibit broadly similar sensitivities to glycine (Pribilla et al., 1992; Yang et al., 2007)
The sensitivity to strychnine in all three age groups did not change with development. This is inconsistent with previous studies in cultured mouse spinal neurons (Tapia and Aguayo, 1998) and in neurons acutely dissociated from the VTA (Ye, 2000). Furthermore, it is worthy noticing that the IC50 for strychnine of PFC neurons of peri-adolescent Sprague-Dawley rats is 130 nM, about 11 times of that in VTA neurons of similar age (P24–40), which have an IC50 of 12 nM (Ye, 2000). These results indicated that in the peri-adolescent PFC neurons, the α1-containing GlyRs are much lower than that of VTA neurons of the same age. This notion is supported by a recent study that used qPCR comparing the relative GlyR expression in the forebrain of Alko Alchol/Non-Alcohol (AA/ANA) rats, which found that the α1 subunit mRNA expression level in the VTA was the highest, but was not detected in the PFC (Jonsson et al., 2009).
Since α2 is the most abundantly expressed α subunit in adult PFC neurons (Jonsson et al., 2009), it would therefore be reasonable to assume that the IGly in the PFC neurons is mediated by the α2 subtypes. The existence of α2 homomers in the PFC neurons is also implied by relative high picrotoxin sensitivity of IGly recorded from these neurons, especially from the peri-adolescent group. In the current study, we found that low concentrations of picrotoxin (30 µM) partially blocked the IGly recorded from PFC neurons, suggesting that a subpopulation of the GlyRs in the PFC neurons is likely to be α2 homomeric receptors- the immature extrasynaptic form. Conversely, the IGly which is resistant to the higher picrotoxin concentrations (>300 µM) may be induced by the activation of the αβ heteromeric GlyRs, which have a much lower sensitivity to picrotoxin (Chattipakorn and McMahon, 2002; Pribilla et al., 1994; Ye, 2000; Yoon et al., 1998). Interestingly, the peri-adolescent group has much higher picrotoxin sensitivity than the weaning and the neonatal groups, suggesting that the GlyRs in PFC neurons of the peri-adolescent group contain more α2 homomeric receptors compared to the other two age groups. The other interpretation for this is that the β subunit decreased with increase of age. Furthermore, since previous studies have shown that the β subunit of GlyRs is required for receptor clustering (Kirsch et al., 1993; Meyer et al., 1995), the finding of possible αβ heteromeric GlyRs in PFC raises the possibility that some GlyRs may be synaptically located. According to the present study, this is more in the neonatal and weaning groups than in the peri-adolescent group. However, additional experiments will need to be undertaken to test this hypothesis.
In summary, results from the present study indicated that: (1) functional GlyRs exist in the PFC neurons of young rats, (2) their physiological and pharmacological properties changed substantially during maturation, and (3) there are at least two subtypes of GlyRs in the PFC neurons.
4. Materials and methods
4.1. Isolation of neurons and electrophysiological recording
PFC neurons were prepared as previously described (Ye and Akaike, 1993). Briefly, Sprague-Dawley rats (P5–P39) were anesthetized with ketamine/xylazine (80 mg/5 mg/kg, IP) and then sacrificed by decapitation. Coronal slices (250 µm thick) containing PFC were cut using a Compresstome™ VF-200 slicer (Precisionary Instruments Inc., Greenville, NC) in ice-cold glycerol-based artificial cerebrospinal fluid (GACSF) containing: 250 mM glycerol, 1.6 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.4 mM CaCl2, 25 mM NaHCO3, and 11 mM glucose, and saturated with 95% O2−5% CO2 (carbogen) (Ye et al., 2006). Slices were then transferred to a standard external solution containing 3 mg/ml protease XIII (Sigma-Aldrich Chemical Company, St Louis, MO, US) and incubated (in 32°C) for 8 min. The standard external solution contained 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM HEPES, and saturated with 100% O2. The pH of the solution was adjusted to 7.4 with Tris Base and the osmolarity to 320 mM/kg with sucrose. When the incubation was completed, these slices were washed six times with the oxygenated standard external solution. Then the PFC was identified according to the stereotaxic coordinates (Paxinos and Watson, 2007), isolated with a blade and transferred to a 10 ml tube filled with oxygenated standard external solution. Mild trituration of these tissues through heat polished pipettes of progressively smaller tip diameter served to dissociate single neurons. A drop of solution containing PFC neurons was transferred to a 35 mm cultured dish. After 20 min, isolated neurons that adhered to the bottom of the dish were used for electrophysiological recordings. The patch electrode had a resistance between 3 and 5 MΩ when filled with solution containing 120 mM CsCl, 21 mM tetraethylammonium chloride, 4 mM MgCl2, 11 mM EGTA, 1 mM CaCl2, 10 mM HEPES, and 2 mM Mg-ATP. The pH was adjusted to 7.2 with Tris-Base and the osmolarity to 280 mM/kg with sucrose.
Whole-cell IGly was recorded in a conventional whole-cell configuration with an Axopatch 1D amplifier (Molecular Devices Co., Union City, CA), a Digidata 1320A digitizer (Molecular Devices Co.) and pClamp 10.2 software (Molecular Devices Co.). Data was filtered at 1 kHz and sampled at 5 kHz. Junction potential was nulled immediately before forming a Gigaseal. PFC contains both pyramidal and interneurons. All recordings were from pyramidal neurons judging by their shape.
4.2. Chemicals and application
Chemicals, including glycine, strychnine, and picrotoxin were purchased from Sigma Aldrich (St. Louis, MO, USA). The solutions of these chemicals were prepared on the day of the experiment. Picrotoxin was dissolved in methanol; the final concentration of methanol in test solution was <0.1% (v/v), which has no effect on IGly (Ye, 2000). The drugs were added to the superfusate and were applied to the cell using a fast perfusion system (Y tube). Solutions in the vicinity of a neuron can be completely exchanged within 40 ms without damaging the seal (Zhou et al., 2006). After each application of the agonist and the antagonist(s) of GlyRs, a > 2 min washout time was followed to ensure that the receptors recovered completely before the next drug application.
4.3 Data analysis
The date was measured by pClamp10.2 (Molecular Devices Co.). Graphics and statistical data analysis were carried out by Sigmaplot 11.0 (SPSS Inc, Chicago, IL, USA). Data is presented as mean ± standard error of the mean (S.E.M) when appropriating. Statistical analysis was performed using one way ANOVA, and significant main effect was further analyzed using Student-Newman-Keuls Method, and values of P<0.05 were considered significant.
Research Highlights.
We show that functional glycine receptors exist in cortical neurons of young rats.
The properties of cortical glycine receptors change with development.
The cortical neurons contain at least two subtypes of glycine receptors.
AKNOWLEDGMENTS
Funding: NIH, NIAAA R01AA016964, R21AA016618, R21AA015925 to JHY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aguayo LG, Pancetti FC. Ethanol modulation of the gamma-aminobutyric acidA- and glycine-activated Cl- current in cultured mouse neurons. J Pharmacol Exp Ther. 1994;270:61–69. [PubMed] [Google Scholar]
- Aguayo LG, van Zundert B, Tapia JC, Carrasco MA, Alvarez FJ. Changes on the properties of glycine receptors during neuronal development. Brain Res Brain Res Rev. 2004;47:33–45. doi: 10.1016/j.brainresrev.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Aprison MH, Werman R. The distribution of glycine in cat spinal cord and roots. Life Sci. 1965;4:2075–2083. doi: 10.1016/0024-3205(65)90325-5. [DOI] [PubMed] [Google Scholar]
- Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. Embo J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci. 1991;14:458–461. doi: 10.1016/0166-2236(91)90045-v. [DOI] [PubMed] [Google Scholar]
- Chattipakorn SC, McMahon LL. Pharmacological haracterization of glycine-gated chloride currents recorded in rat hippocampal slices. J Neurophysiol. 2002;87:1515–1525. doi: 10.1152/jn.00365.2001. [DOI] [PubMed] [Google Scholar]
- Flint AC, Liu X, Kriegstein AR. Nonsynaptic glycine receptor activation during early neocortical development. Neuron. 1998;20:43–53. doi: 10.1016/s0896-6273(00)80433-x. [DOI] [PubMed] [Google Scholar]
- Frostholm A, Rotter A. Glycine receptor distribution in mouse CNS: autoradiographic localization of [3H]strychnine binding sites. Brain Res Bull. 1985;15:473–486. doi: 10.1016/0361-9230(85)90038-3. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger ED, Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Schmieden V, Schofield PR, Seeburg PH, Siddique T, Mohandas TK, Becker CM, Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. Embo J. 1990;9:771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Kerekes N, Hyytia P, Ericson M, Soderpalm B. Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res. 2009 doi: 10.1016/j.brainres.2009.09.053. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Kungel M, Friauf E. Physiology and pharmacology of native glycine receptors in developing rat auditory brainstem neurons. Brain Res Dev Brain Res. 1997;102:157–165. doi: 10.1016/s0165-3806(97)00087-4. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Malosio ML, Marqueze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. Embo J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin JM, Guyon A, Eugene D, Paupardin-Tritsch D, Legendre P. Functional glycine receptor maturation in the absence of glycinergic input in dopaminergic neurones of the rat substantia nigra. J Physiol. 2002;542:685–697. doi: 10.1113/jphysiol.2002.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Li KY, daGraca RL, Delphin E, Xiong M, Ye JH. Behavior and cellular evidence for propofol-induced hypnosis involving brain glycine receptors. Anesthesiology. 2009;110 doi: 10.1097/ALN.0b013e3181942b5b. 326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat brain in stereotaxic coordinates 6th edition. Academic press; 2007. [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. Embo J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. Embo J. 1994;13:1493. doi: 10.1002/j.1460-2075.1994.tb06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Tohyama M. Regional distribution of cells expressing glycine receptor alpha 2 subunit mRNA in the rat brain. Brain Res. 1992;590:95–108. doi: 10.1016/0006-8993(92)91085-s. [DOI] [PubMed] [Google Scholar]
- Schmieden V, Grenningloh G, Schofield PR, Betz H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. Embo J. 1989;8:695–700. doi: 10.1002/j.1460-2075.1989.tb03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia JC, Aguayo LG. Changes in the properties of developing glycine receptors in cultured mouse spinal neurons. Synapse. 1998;28:185–194. doi: 10.1002/(SICI)1098-2396(199803)28:3<185::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Watanabe E, Akagi H. Distribution patterns of mRNAs encoding glycine receptor channels in the developing rat spinal cord. Neurosci Res. 1995;23:377–382. doi: 10.1016/0168-0102(95)00972-V. [DOI] [PubMed] [Google Scholar]
- Yang Z, Cromer BA, Harvey RJ, Parker MW, Lynch JW. A proposed structural basis for picrotoxinin and picrotin binding in the glycine receptor pore. J Neurochem. 2007;103:580–589. doi: 10.1111/j.1471-4159.2007.04850.x. [DOI] [PubMed] [Google Scholar]
- Ye J. Physiology and pharmacology of native glycine receptors in developing rat ventral tegmental area neurons. Brain Res. 2000;862:74–82. doi: 10.1016/s0006-8993(00)02073-4. [DOI] [PubMed] [Google Scholar]
- Ye JH, Akaike N. Calcium currents in pyramidal neurons acutely dissociated from the rat frontal cortex: a study by the nystatin perforated patch technique. Brain Res. 1993;606:111–117. doi: 10.1016/0006-8993(93)91577-f. [DOI] [PubMed] [Google Scholar]
- Ye JH, Tao L, Ren J, Schaefer R, Krnjevic K, Liu PL, Schiller DA, McArdle JJ. Ethanol potentiation of glycine-induced responses in dissociated neurons of rat ventral tegmental area. J Pharmacol Exp Ther. 2001;296:77–83. [PubMed] [Google Scholar]
- Ye JH, Zhang J, Xiao C, Kong JQ. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods. 2006;158:251–259. doi: 10.1016/j.jneumeth.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Ye JH, Sokol KA, Bhavsar U. Glycine receptors contribute to hypnosis induced by ethanol. Alcoholism: Clinical and Experimental Research. 2009;33 doi: 10.1111/j.1530-0277.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KW, Wotring VE, Fuse T. Multiple picrotoxinin effect on glycine channels in rat hippocampal neurons. Neuroscience. 1998;87:807–815. doi: 10.1016/s0306-4522(98)00158-4. [DOI] [PubMed] [Google Scholar]
- Young AB, Snyder SH. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973;70:2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AB, Snyder SH. The glycine synaptic receptor: evidence that strychnine binding is associated with the ionic conductance mechanism. Proc Natl Acad Sci U S A. 1974;71:4002–4005. doi: 10.1073/pnas.71.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Xiao C, McArdle JJ, Ye JH. Mefloquine enhances nigral gamma-aminobutyric acid release via inhibition of cholinesterase. J Pharmacol Exp Ther. 2006;317:1155–1160. doi: 10.1124/jpet.106.101923. [DOI] [PubMed] [Google Scholar]