Abstract
A scaffold hopping strategy was employed to identify new chemotypes that inhibit noroviruses. The replacement of the cyclosulfamide scaffold by an array of heterocyclic scaffolds lead to the identification of additional series of compounds that possessed anti-norovirus activity in a cell-based replicon system.
Introduction
Noroviruses are single-stranded RNA, non-enveloped viruses that belong to the Norovirus genus of the Caliciviridae family. They are the cause of ~21 million cases of acute gastroenteritis in the U.S.1 Noroviruses are highly contagious, consequently, outbreaks of acute gastroenteritis are common, particularly in schools, nursing homes, restaurants, hospitals, and cruise ships. Currently, there is no vaccine or low molecular weight antiviral drug available for the treatment of norovirus infections.
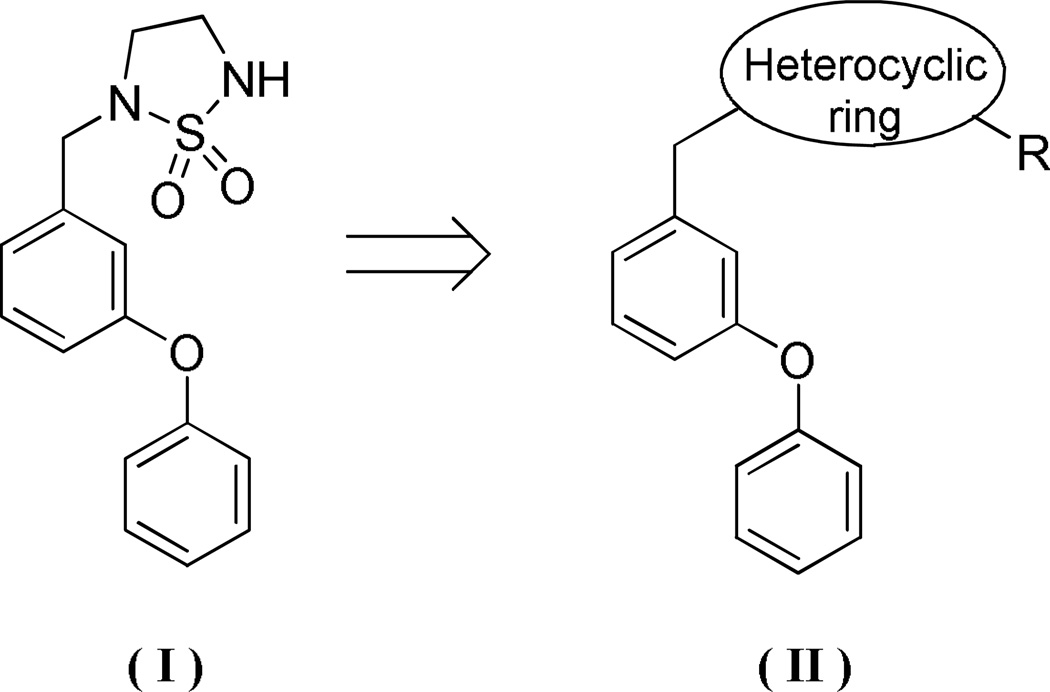
Scaffold hopping (also termed chemotype switching)2–3 is an integral component of the drug discovery process and is an effective strategy for exploring chemical space and the discovery of back-up series of compounds. This is a powerful approach for identifying new chemotypes which display improved pharmacological and ADME/Tox characteristics, as well as address intellectual property issues. We have recently described the discovery of a new class of anti-norovirus agents that embody in their structure the cyclosulfamide scaffold (Figure 1). We describe herein the results of our studies related to the application of scaffold hopping in the discovery of new series of compounds that inhibit noroviruses by modifying the cyclosulfamide core structure (Figure 1).
Figure 1.
Norovirus inhibitor scaffold hopping strategy.
Chemistry
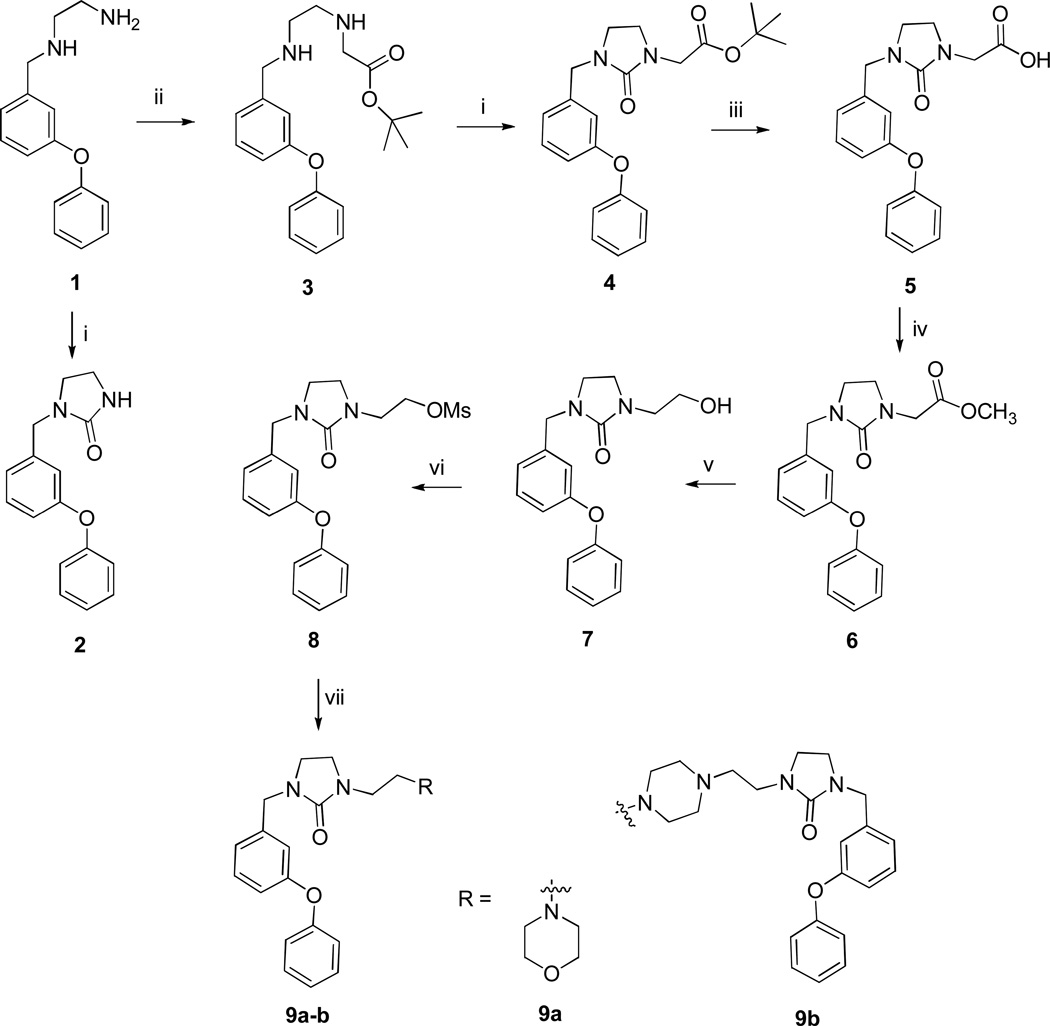
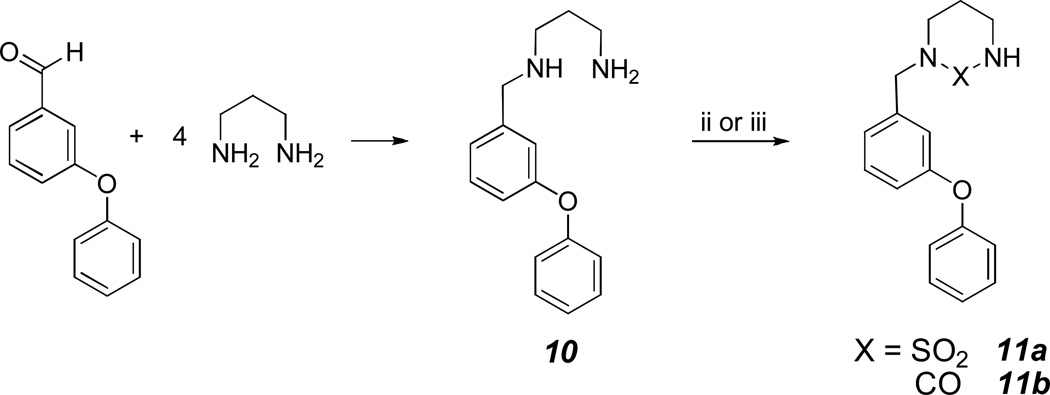
Compounds 2, 4–5 and 9a–b were synthesized as shown in Scheme 1. These compounds were readily synthesized by reductive amination of m-(phenoxy)benzaldehyde with excess ethylenediamine in the presence of sodium borohydride in methanol4–5 to yield the corresponding N-substituted ethylene diamine 1. Stirring overnight with carbonyldiimidazole in dioxane yielded N-substituted imidazolidinone 2. Alkylation of 2 with sodium hydride followed by methyl or t-butyl bromoacetate gave low yields of the corresponding products, consequently an alternative method involving refluxing N-(m-phenoxy)benzylethylene diamine 1 and t-butyl bromoacetate in DMF was used.6 Compound 3 was cyclized to the corresponding N-substituted imidazolidinone with carbonyldiimidazole. Teatment with TFA followed by esterification and lithium borohydride reduction of the resulting ester gave alcohol 7. Formation of mesylate 8 followed by refluxing with morpholine in 95% ethanol in the presence of sodium bicarbonate yielded 9a. Refluxing 8 with piperazine gave the corresponding dimer 9b. Compounds 11a–b were made by refluxing (m-phenoxy)benzaldehyde and 1,3-diaminopropane to yield intermediate 10, which was converted to compounds 11a and 11b by refluxing with sulfamide in pyridine or stirring with carbonyldiimidazole, respectively (Scheme 2).
Scheme 1.
Reaction conditions: i) CDI/1,4-dioxane; ii) BrCH2COOC(CH3)3/DMF/0°C to rt; iii) TFA; iv) SOCl2/CH3OH; v) LiBH4/THF/EtOH; vi) MsCl/TEA/CH2Cl2; vii) 1eq morpholine or 0.5 eq piperazine, NaHCO3/95% EtOH/reflux.
Scheme 2.
Reaction conditions: i) NaBH4/MeOH; ii) NH2SO4NH2/pyridine/reflux 16h; iii) CDI/1,4-dioxance
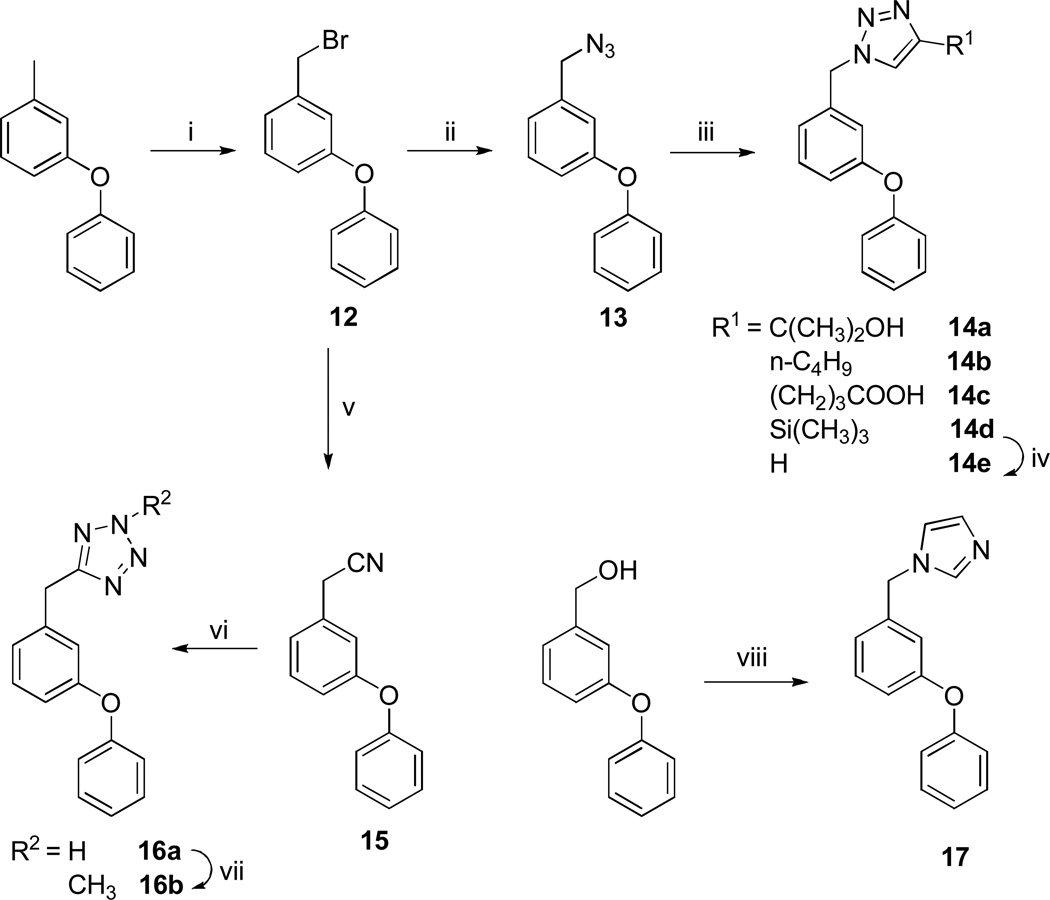
Triazole derivatives 14a–e were readily synthesized using click chemistry7–8 as illustrated in Scheme 3. Tetrazole derivatives 16a-b were prepared by heating nitrile 15 with sodium azide in DMF (Scheme 3). Imidazole derivative 17 was readily synthesized by reacting (m-phenoxy)benzyl alcohol with carbonyldiimidazole in acetonitrile9 (Scheme 3).
Scheme 3.
Reaction conditions: i) NBS/AIBN/CCl4; ii) NaN3/DMSO; iii) R1C≡ CH/Sodium ascorbate/CuSO4/t-BuOH:H2O(1:1) or CuI/DMSO; iv) TBAF/THF; v) NaCN/DMSO; vi) NaN3/NH4Cl/DMF/100 °C; vii) CH3I/TEA/ACN; viii) CDI/THF/ACN.
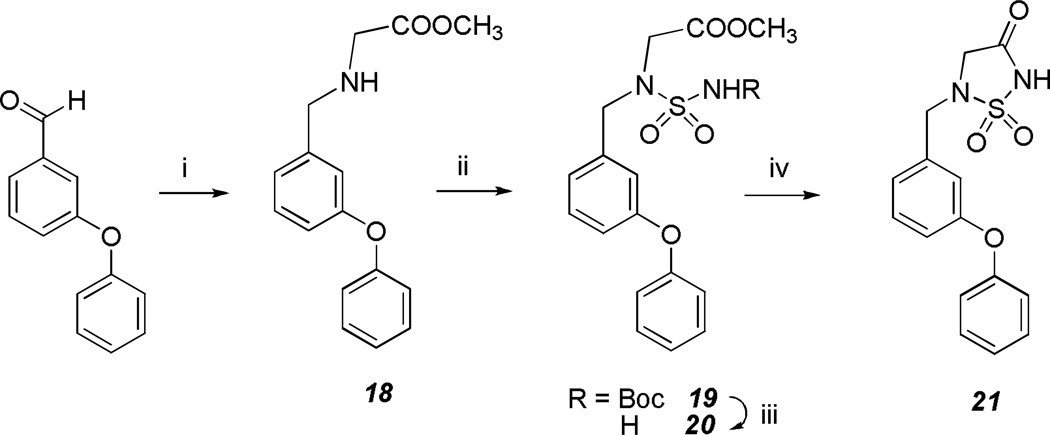
Compound 21 was synthesized as illustrated in Scheme 4 using similar procedures as those described previously.10–12
Scheme 4.
Reaction conditions: i) Gly-OCH3(HCl)/TEA/NaBH4/CH3OH; ii) a) ClSO2NCO/t-BuOH/CH2Cl2, b) TEA/CH2Cl2; iii) TFA; iv) NaH/THF.
Biochemical Studies
The effects of the synthesized compounds were examined in NV replicon-harboring cells (HG23 cells) and the results are summarized in Table 1. Detailed procedures for studying the antiviral effects using HG23 cells have been reported elsewhere.13–15
Table 1.
| Compound | ED50 (µM) | TD50 (µM) |
|---|---|---|
| 2 | 8 | 95 |
| 4 | 12 | 55 |
| 5 | >20 | ND |
| 9a | 16 | 120 |
| 9b | 0.5 | 2.5 |
| 11a | > 20 | ND |
| 11b | > 20 | ND |
| 14a | > 20 | ND |
| 14b | 6 | 40 |
| 14c | > 20 | ND |
| 14d | 7 | 30 |
| 14e | 10 | 100 |
| 16a | > 20 | ND |
| 16b | > 20 | ND |
| 17 | 8 | 35 |
| 21 | >20 | ND |
ND: not determined due to high ED50 value
Results and Discussion
Noroviruses constitute a significant public health problem. There are currently no drugs on the market for the treatment of norovirus infection and, furthermore, only a limited number of studies have been reported in the literature related to the development of norovirus therapeutics.16–18 Using a cell-based replicon system, we have recently demonstrated that cyclosulfamide-based derivatives are potent inhibitors of noroviruses (Figure 1, structure (I)). Furthermore, structure-activity relationship studies indicated that anti-norovirus activity was greatly influenced by multiple factors, including the nature of the groups attached to the cyclosulfamide scaffold and the nature of the rings in the diphenyl ether moiety. Based on these findings, we have used the cyclosulfamide scaffold as the starting point of a scaffold hopping strategy aimed at identifying new chemotypes that possess enhanced binding affinity and aqueous solubility, as well as other drug-like characteristics.19
A conservative change involving the replacement of the SO2 moiety in cyclosulfamide by C=O to yield a 2-imidazolidinone ring was initially made (compound 2). The potency of compound 2 was lower than that of the corresponding cyclosulfamide compound, however, there was a small improvement in the TD50 (Table 1). The 2-imidazolidinone scaffold was also embellished with an acidic (compound 5) or basic (compound 9a) component to increase aqueous solubility. These compounds were either inactive (compound 5) or exhibited reduced anti-norovirus activity (compound 9a). In contrast, dimer 9b was an order of magnitude more potent than the initial cyclosulfamide hit, however, it had high toxicity (Table 1). Increasing the ring size of the cyclosulfamide or 2-imidazolidinone rings yielded compounds that were devoid of anti-norovirus activity (compounds 11a and 11b, Table 1).
We envisaged that the replacement of the cyclosulfamide ring with a series of structurally-diverse electron-rich rings may yield compounds exhibiting greater affinity with the putative receptor. Thus, the cyclosulfamide scaffold was sequentially replaced by a triazole, tetrazole, imidazole or 1, 2, 5-thiadiazolidin-3-one 1,1 dioxide ring. A few of the triazole derivatives (compounds 14b, 14d and 14e, Table 1) exhibited anti-norovirus activity, however, their TD50 values were too low. In both the 2-imidazolidinone and triazole series (compounds 5 and 14c, Table 1), the presence of a carboxyl group was inimical to anti-norovirus activity. The tetrazole derivatives were inactive (compounds 16a and 16b, Table 1), while the corresponding imidazole compound was moderately active with a low therapeutic index. Replacement of the cyclosulfamide ring with the 1,2,5-thiadiazolidin-3-one 1,1 dioxide scaffold yielded an inactive compound (compound 21, (Table 1). Taken together, these observations suggest that that the nature of the heterocyclic ring has a profound effect on the anti-norovirus activity and cytotoxicity of these compounds.
In conclusion, a scaffold hopping strategy was employed to identify new chemotypes that inhibit noroviruses. These preliminary studies suggest that pharmacological activity and cytotoxicity are impacted by subtle changes in structure. Identification of the molecular target(s) these compounds interact with should greatly facilitate exploitation of these observations and may lead to the emergence of effective anti-norovirus therapeutics.
Experimental Section
General
The 1H spectra were recorded on a Varian XL-300 or XL-400 NMR spectrometer. Melting points were determined on a Mel-Temp apparatus and are uncorrected. Reagents and solvents were purchased from various chemical suppliers (Aldrich, Acros Organics, TCI America, and Bachem). Silica gel (230–450 mesh) used for flash chromatography was purchased from Sorbent Technologies (Atlanta, GA). Thin layer chromatography was performed using Analtech silica gel plates to determine the compound purity. The TLC plates for all the compounds were eluted using two different solvent systems and visualized using iodine and/or UV light. Each individual compound was identified as a single spot on TLC plate (purity greater than 95%).
Representative synthesis
1-(3-Phenoxybenzyl)imidazolidin-2-one (2)
To a solution of ethylenediamine (6.00 g; 100 mmol) in 65 mL methanol kept in an ice bath was added 3-(phenoxy)benzaldehyde (4.95 g; 25 mmol) in small portions. After the addition, sodium borohydride (0.94 g; 25 mmol) was slowly added portionwise at 0 °C. The reaction was allowed to warm to room temperature overnight with stirring. The solvent was removed and the residue was taken up in ethyl acetate (100 mL). The organic layer was washed with water (40 mL) and dried over anhydrous sodium sulfate. The drying agent was filtered off and the solvent was removed in vacuo to give pure compound 1 as colorless oil (6.00 g; 99% yield). 1H NMR (CDCl3): δ 1.40 (s, 3H), 2.65- 2.90 (m, 4H), 3.78 (s, 2H), 6.98 (t, J = 10.1 Hz, 3H), 7.10 (t, J = 9.8 Hz, 1H), 7.20–7.40 (m, 5H). To solution of compound 1 (0.52 g; 2 mmol) in dry 1,4-dioxane (12 mL) was added a solution of N,N′-carbonyldiimidazole (0.40 g; 2.48 mmol) in 2 mL dry 1,4-dioxane. The reaction mixture was stirred at room temperature for 18 h. The solvent was removed and the residue was taken up in ethyl acetate (20 mL). The organic layer was washed with 5% HCl (3 × 10 mL), brine (10 mL) and then dried over anhydrous sodium sulfate. The drying agent was filtered off and the solvent was evaporated to give a white solid 2 (0.32 g; 56% yield), mp 108–109 °C. 1H NMR (CDCl3): δ 3.28–3.43 (m, 4H), 4.37 (s, 2H), 5.54 (s, 1H), 6.89–7.37 (m, 9H). HRMS (ESI) calculated m/z for C16H16N2O2Na [M+Na]+ 291.1109; found 291.1084.
tert-Butyl 2-(2-(3-phenoxybenzylamino)ethylamino)acetate (3)
To a solution of t-butyl 2-bromoacetate (9.84 g; 66.6 mmol) in dry DMF (90 mL) kept in an ice bath was added dropwise a solution of N-(m-phenoxy)benzyl ethylenediamine (48.00 g; 200 mmol) in dry DMF (600 mL) over 1.5 h. After the addition, the reaction was allowed to warm to room temperature and stirred for 16 h. DMF was removed under vacuum and the residue was taken up in ethyl acetate (500 mL) and water (400 mL). The organic layer was separated and the aqueous solution was extracted with an additional 200 mL of ethyl acetate. The combined organic extracts were dried over anhydrous sodium sulfate. The drying agent was filtered off and the solvent was removed on the rotary evaporator. The crude product was purified using flash chromatography (silica gel/ethyl acetate/hexanes) to give compound 3 as a yellow oil (25.40 g; 98% yield). 1H NMR (CDCl3): δ 1.43 (s, 9H), 2.74 (s, 4H), 3.39 (s, 2H), 3.80 (s, 2H), 6.85–7.40 (m, 9H).
tert-Butyl 2-(2-oxo-3-(3-phenoxybenzyl)imidazolidin-1-yl)acetate (4)
Compound 4 was prepared using the same procedure as that used in the synthesis of compound 2. Yellow oil (76% yield). 1H NMR (CDCl3): δ 1.44 (s, 9H), 3.20–3.28 (m, 2H), 3.39–3.47 (m, 2H), 3.90 (s, 2H), 4.38 (s, 2H), 6.85–7.36 (m, 9H). HRMS (ESI) calculated m/z for C22H27N2O4 [M+H]+ 383.1971; found 383.1968.
2-(2-Oxo-3-(3-phenoxybenzyl)imidazolidin-1-yl)acetic acid (5)
Compound 4 (19.00 g; 49.7 mmol) was treated with trifluoroacetic acid (150 mL) and stirred for 1 h. TFA was removed and the pH of the residue was adjusted to 10 using cold 1N NaOH. The aqueous solution was extracted with ethyl acetate (2 × 100 mL) to remove unreacted starting material and the pH of the aqueous layer was adjusted to ~1 with 6 N HCl. The solution was extracted with ethyl acetate (2 × 100 mL) and the combined organic layers were dried over anhydrous sodium sulfate. The drying agent was filtered off and the solvent was removed to give pure compound 5 as a yellow oil (15.68 g; 97% yield). 1H NMR (CDCl3): δ 3.31–3.36 (m, 2H), 3.48–3.53 (m, 2H), 4.05 (s, 2H), 4.38 (s, 2H), 6.88–7.37 (m, 9H), 9.90 (s, 1H). HRMS (ESI) calculated m/z for C18H19N2O4 [M+H]+ 327.1345; found 327.1359.
Methyl 2-(2-oxo-3-(3-phenoxybenzyl)imidazolidin-1-yl)acetate (6)
To 10 mL dry methanol kept in an ice bath was added dropwise thionyl chloride (1.31 g; 11 mmol), followed by the addition of compound 5 (3.59 g; 11 mmol) in small portions. After the addition, the reaction mixture was warmed to 40 °C for 2 h. The solvent was removed under vacuum and the residue was dissolved in 20 mL ethyl acetate. The solvent was again removed under vacuum to give compound 6 as a colorless oil (3.40 g; 100% yield). 1H NMR (CDCl3): δ 3.23–3.32 (m, 2H), 3.40–3.48 (m, 2H), 3.75 (s, 3H), 4.02 (s, 2H), 4.39 (s, 2H), 6.85–7.36 (m, 9H).
1-(2-Hydroxyethyl)-3-(3-phenoxybenzyl)imidazolidin-2-one (7)
To a solution of compound 6 (1.70 g; 5 mmol) in 8 mL dry THF was added dropwise a solution of 2 M LiBH4 (2.5 mL; 5 mmol), followed by dropwise addition of absolute ethanol (15 mL). The reaction mixture was stirred at room temperature overnight. The reaction mixture was cooled in an ice bath and acidified with 5% aqueous HCl to pH 4. The solvent was removed under vacuum and the residue was taken up in ethyl acetate (85 mL) and washed with brine (25 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent was removed under vacuum to give compound 7 as a colorless oil (1.25 g; 80% yield). 1H NMR (CDCl3): δ 3.20–3.42 (m, 7H), 3.77 (t, J = 5.1 Hz, 2H), 4.35 (s, 2H), 6.85–7.36 (m, 9H).
2-(2-Oxo-3-(3-phenoxybenzyl)imidazolidin-1-yl)ethyl methanesulfonate (8)
To a solution of compound 7 (1.25 g; 4 mmol) and triethylamine (0.41 g; 4 mmol) in 10 ml dry methylene chloride was added methanesulfonyl chloride (0.50 g; 4.3 mmol) at 0 °C. The reaction mixture was allowed to warm to room temperature and stirred overnight. Methylene chloride (10 mL) was added to the reaction mixture and the resulting solution was washed with saturated sodium bicarbonate (2 × 20 mL). The organic layer was separated and dried over anhydrous sodium sulfate. The drying agent was filtered off and the solvent was removed to give compound 8 as a colorless oil (1.56 g; 100% yield). 1H NMR (CDCl3): δ 3.02 (s, 3H), 3.25 (t, J = 7.6 Hz, 2H), 3.45 (t, J = 7.6 Hz, 2H), 3.58 (t, J = 4.8 Hz, 2H), 4.35–4.40 (m, 4H), 6.88–7.37 (m, 9H).
1-(2-Morpholinoethyl)-3-(3-phenoxybenzyl)imidazolidin-2-one (9a)
A mixture of compound 8 (0.86 g; 2.2 mmol), morpholine (0.19 g; 2.2 mmol) and NaHCO3 (1.0 g; 12 mmol) in 10 ml 95% ethanol was refluxed overnight. The solvent was removed and the residue was taken up in ethyl acetate (30 mL) and water (30 mL). The organic layer was separated, washed with 30 mL brine and then dried over anhydrous sodium sulfate. The drying agent was filtered off and the solvent was removed to give pure compound 9a as a white solid (0.65 g; 78% yield), mp 66–68 °C. 1H NMR (CDCl3): δ 2.43–2.54 (m, 6H), 3.17–3.23 (m, 2H), 3.32–3.41 (m, 4H), 3.70 (t, J = 4.9 Hz, 4H), 4.36 (s, 2H), 6.86–7.37 (m, 9H). HRMS (ESI) calculated m/z C22H28N3O3 [M+H]+ 382.2131; found 382.2151.
3,3'-(2,2'-(Piperazine-1,4-diyl)bis(ethane-2,1-diyl))bis(1-(3-phenoxybenzyl)imidazo-lidin-2-one) (9b)
Compound 9b was prepared using a similar procedure as that used for making compound 9a using piperazine. Colorless oil (55% yield). 1H NMR (CDCl3): δ 2.40–2.60 (m, 12H), 3.12–3.21 (m, 4H), 3.28–3.43 (m, 8H), 4.34 (s, 4H), 6.82–7.37 (m, 18H). HRMS (ESI) calculated m/z C40H47N6O4 [M+H]+ 675.3659; found 675.3645.
N1-(3-Phenoxybenzyl)propane-1,3-diamine (10)
To a solution of 1, 3-diaminopropane (7.4 g; 100 mmol) in 65 mL methanol kept in an ice bath was added 3-(phenoxy)benzaldehyde (4.95 g; 25 mmol) in small portions. After the addition, sodium borohydride (0.94 g; 25 mmol) was added slowly in small portions at 0 °C. The reaction was allowed to warm to room temperature overnight with stirring. The solvent was removed and the residue was taken up in ethyl acetate (50 mL), and water (40 mL) was added. The two layers were separated and the organic layer was washed with water (40 mL) and dried over anhydrous sodium sulfate. The drying agent was filtered off and the solvent was removed to give pure compound 10 as a colorless oil (6.40 g; 100% yield). 1H NMR (CDCl3): δ 1.38 (s, 3H), 1.64 (t, J = 7.5 Hz, 2H), 2.66 (t, J = 7.0 Hz, 2H), 2.74 (t, J = 7.0 Hz, 2H), 3.78 (s, 2H), 6.82–7.35 (m, 9H).
2-(3-Phenoxybenzyl)-1,2,6-thiadiazinane 1,1-dioxide (11a)
To a refluxing solution of sulfamide (0.48 g; 5 mmol) in anhydrous pyridine (12 mL) was slowly added compound 10 (1.28 g; 5 mmol) over 1h. The resulting reaction mixture was refluxed for an additional 16 h. Pyridine was removed under vacuum, and the residue was taken up in ethyl acetate (20 mL). The organic layer was washed with 5% HCl (3 × 10 mL), brine (10 mL) and then dried over anhydrous sodium sulfate. The drying agent was filtered off and the solvent was removed, leaving a crude product which was purified using flash chromatography (silica gel/ethyl acetate/hexanes) to give pure compound 11a as a white solid (1.45 g; 91% yield), mp 94–96 °C. 1H NMR (CDCl3): δ 1.60–1.71 (m, 2H), 3.20 (t, J = 5.6 Hz, 2H), 3.49 (q, J = 6.4 Hz, 2H), 4.19 (s, 2H), 4.57 (t, J = 7.1 Hz, 1H), 6.88–7.38 (m, 9H). HRMS (ESI) calculated m/z for C16H19N2O3S [M+H]+ 319.1116; found 319.1129.
1-(3-Phenoxybenzyl)tetrahydropyrimidin-2(1H)-one (11b)
To solution of compound 10 (0.52 g; 2 mmol) in dry 1, 4-dioxane (12 mL) was added a solution of N,N′-carbonyldiimidazole (0.40 g; 2.48 mmol) in 2 mL dry 1,4-dioxane. The reaction mixture was stirred at room temperature for 18 h. The solvent was removed and the residue was taken up in ethyl acetate (20 mL). The organic layer was washed with 5% HCl (3 × 10 mL), brine (10 mL) and dried over anhydrous sodium sulfate. The drying agent was filtered off and the solvent was removed to give a solid. The crude product was washed with 20 mL diethyl ether to give pure compound 11b as a white solid (0.32 g; 57% yield), mp 91–93 °C. 1H NMR (CDCl3): δ 1.80–1.92 (m, 2H), 3.19 (t, J = 5.1 Hz, 2H), 3.30 (t, J = 4.8 Hz, 2H), 4.53 (s, 2H), 6.80–7.40 (m, 9H). HRMS (ESI) calculated m/z for C17H19N2O2 [M+H]+ 283.1447; found 283.1429.
1-(Azidomethyl)-3-phenoxybenzene (13)
A solution of m-(phenoxy)toluene (3.68 g; 20 mmol) in 45 mL CCl4 was treated with N-bromosuccinimide (5.34 g; 30 mmol) and azo-bis(isobutyronitrile) (15 mg) and the reaction mixture was refluxed for 3 h. The solution was allowed to cool to room temperature and then placed in an ice bath. A white precipitate formed which was filtered off and the filtrate was evaporated, leaving pure compound 1 as a yellow oil (4.5 g; 86% yield). 1H NMR (CDCl3): δ 4.4 (s, 2H), 6.9–7.41 (m, 9H). This was used in the next step. To sodium azide (3.25 g; 50 mmol) in dry DMSO (80 mL) was added compound 12 (4.5 g; 17.1 mmol) and the reaction mixture was stirred overnight at room temperature. The reaction mixture was cooled in an ice bath and quenched with water (50 mL). The aqueous layer was extracted with ethyl ether (3 × 30 mL). The combined ethyl ether extracts were washed with water (2 × 20 mL) and dried over anhydrous sodium sulfate. The solvent was removed, leaving a crude product which was purified using flash chromatography (silica gel/methylene chloride/ hexanes) to give compound 13 as a colorless oil (3.31 g; 60% yield). 1H NMR (CDCl3): δ 4.25 (s, 2H), 6.07–7.45 (m, 9H).
2-(1-(3-Phenoxybenzyl)-1H-1,2,3-triazol-4-yl)propan-2-ol (14a)
To compound 13 (2.02 g; 9 mmol) was added 2-methyl-3-butyn-2-ol (0.76 g; 9 mmol), t-butyl alcohol (20 mL), water (20 mL), sodium ascorbate (0.3 g; 1.5 mmol), and CuSO4.5H2O (60 mg). The reaction mixture was stirred overnight at ~ 50°C. Cold water (40 mL) was added, whereupon a solid formed. The solid was collected by filtration and purified using flash chromatography (silica gel/ethyl acetate/hexanes) to give compound 14a as light yellow colored oil (0.40 g; 15% yield). 1H NMR (CDCl3): δ 1.60 (s, 6H), 2.40 (s, 1H), 5.46 (s, 2H), 6.90–7.05 (m, 4H), 7.10–7.40 (m, 6H). HRMS (ESI) calculated m/z for C18H20N3O2 [M+H]+ 310.1556; found 310.1551.
Compounds 14b-c was prepared using a similar procedure as that described above.
4-Butyl-1-(3-phenoxybenzyl)-1H-1,2,3-triazole (14b)
yellow oil (26% yield). 1H NMR (CDCl3): δ 0.90 (t, J = 10.5Hz, 3H), 1.30–1.42 (m, 2H), 1.60–1.70 (m, 2H), 2.70 (t, J = 10.5Hz, 2H), 5.43 (s, 2H), 6.90–7.05 (m, 4H), 7.10–7.40 (m, 6H). HRMS (ESI) calculated m/z for C19H22N3O [M+H]+ 308.1763; found 308.1742.
4-(1-(3-Phenoxybenzyl)-1H-1,2,3-triazol-4-yl)butanoic acid (14c)
white solid (21% yield). mp 75–77°C. 1H NMR (CDCl3): δ 1.98–2.09 (p, J = 13.3Hz, 2H), 2.50 (t, J = 6.6Hz, 2H), 2.80 (t, J = 6.6Hz, 2H), 5.42 (s, 2H), 6.98–7.40 (m, 10H), 12.02 (s, 1H). HRMS (ESI) calculated m/z for C19H20N3O3 [M+H]+ 338.1505; found 338.1479.
1-(3-Phenoxybenzyl)-4-(trimethylsilyl)-1H-1,2,3-triazole (14d)
A solution of compound 2 (2.25 g; 10 mmol) in 10 mL dry DMSO was treated with trimethylsilyl acetylene (0.98 g; 10 mmol), and CuI (191 mg), and the reaction mixture was heated to 90 °C for 3.5 h. Ethyl acetate (75 mL) was added and the solution was washed with saturated ammonium chloride (100 mL) containing concentrated ammonium hydroxide (2 mL). The layers were separated and the aqueous layer was extracted with ethyl acetate (2 × 100 mL). The combined organic extracts were dried over anhydrous sodium sulfate. The mixture was filtered and the solvent was removed, leaving a crude product as a brown oil which was purified by flash chromatography (silica gel/ethyl acetate/hexanes) to give compound 14d as a light yellow oil (0.70g ; 21% yield). 1H NMR (CDCl3): δ 0.30 (d, J = 10.7Hz, 9H), 5.50 (s, 2H), 6.85 (d, J = 10.7Hz, 1H), 6.90–7.40 (m, 8H), 7.84 (s, 1H). HRMS (ESI) calculated m/z for C18H22N3OSi [M+H]+ 324.1532; found 324.1510.
1-(3-Phenoxybenzyl)-1H-1,2,3-triazole (14e)
Compound 14d (0.48 g; 1.5 mmol) in dry THF (6 mL) was treated with tetra n-butylammonium fluoride (0.39 g; 1.5 mmol) and the reaction mixture was stirred overnight at room temperature. The solvent was removed and the residue was partitioned between saturated ammonium chloride (8 mL) and ethyl acetate (40 mL). The layers were separated and the aqueous layer was extracted with ethyl acetate (2 × 50 mL). The combined organic layers were dried using anhydrous sodium sulfate. The solvent was removed to yield compound 14e as a yellow oil (0.31 g; 83% yield). 1H NMR (CDCl3): δ 5.50 (s, 2H), 6.85 (d, J = 10.7Hz, 1H), 6.90–7.40 (m, 8H), 7.50 (s, 1H), 7.84 (s, 1H). HRMS (ESI) calculated m/z for C15H13N3ONa [M+Na]+ 274.0956; found 274.0942.
2-(3-Phenoxyphenyl)acetonitrile (15)
A solution of compound 12 (5.26 g; 20 mmol) in dry DMSO (5 mL) was added dropwise to a rapidly stirred mixture of sodium cyanide (1.06; 21.6 mmol) in 10 mL DMSO and the reaction mixture was stirred for 5 h at room temperature. Water (50 mL) was added and the solution was extracted with ethyl acetate (3 × 85 mL). The combined organic layers were washed with 30 mL brine and dried over anhydrous sodium sulfate. The solution was filtered and the solvent was evaporated to yield a light yellow oil which was purified by flash chromatography to give compound 15 as a colorless oil (0.90 g; 22% yield). 1H NMR (DMSO-d6): δ 4.31 (s, 2H), 6.82–7.08 (m, 5H), 7.15 (t, J = 10.7Hz, 1H), 7.30–7.41 (m, 4H).
5-(3-Phenoxybenzyl)-2H-tetrazole (16a)
To a stirred solution of compound 15 (2.1 g; 10 mmol) in dry DMF (12 mL) was added ammonium chloride (0.54 g; 10 mmol) and sodium azide (0.64 g; 10 mmol). The suspension was stirred for 8h at 100°C. The reaction mixture was cooled to room temperature and filtered. The filtrate was concentrated to leave a crude product which was treated with water (5 mL) and the solution acidified with 5% HCl. The solution was extracted with ethyl acetate (3 × 50 mL). The combined organic extracts were washed with 30 mL brine and dried over anhydrous sodium sulfate. The solvent was removed to yield a crude product which was purified by flash chromatography to give compound 16a as a light yellow oil (0.70 g; 28% yield). 1H NMR (DMSO-d6): δ 4.31 (s, 2H), 6.82–7.08 (m, 5H), 7.15 (t, J = 10.7Hz, 1H), 7.30–7.41 (m, 4H). HRMS (ESI) calculated m/z for C14H13N4O [M+H]+ 253.1089; found 253.1064.
2-Methyl-5-(3-phenoxybenzyl)-2H-tetrazole (16b)
A solution of compound 16a (0.37 g; 1.5 mmol) and triethylamine (0.17 g; 1.7 mmol) in dry acetonitrile (7 mL) was treated with methyl iodide (0.21 g; 1.5 mmol) with stirring. The reaction mixture was stirred at room temperature for 5 h and refluxed for 20 h. The solution was filtered and the solvent was removed to give compound 16b as a colorless viscous oil (0.34 g; 87% yield). 1H NMR (CDCl3): δ 3.83 (s, 3H), 4.31 (s, 2H), 6.80–7.20 (m, 6H), 7.21–7.40 (m, 3H). HRMS (ESI) calculated m/z for C15H15N4O [M+H]+ 267.1246; found 267.1230.
1-(3-Phenoxybenzyl)-1H-imidazole (17)
3-(Phenoxy)benzyl alcohol (2 g; 10 mmol) and N,N’carbonyldiimidazole (2.42 g; 14 mmol) were dissolved in THF (10 mL) and acetonitrile (14 mL). The reaction mixture was refluxed for 7h. The solvent was removed and the residue was taken up in ethyl acetate (80 mL) and washed with water (30 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent was removed to yield a crude product which was purified by flash chromatography (silica gel/ ethyl acetate/ hexanes) to give compound 17 as a colorless oil (0.6 g; 24% yield). 1H NMR (CDCl3): δ 5.12 (s, 2H), 6.80–7.01 (m, 5H), 7.05–7.20 (m, 2H), 7.26–7.40 (m, 4H), 7.55 (s, 1H). HRMS (ESI) calculated m/z for C16H15N2O [M+H]+ 251.1184; found 251.1169.
Methyl 2-(3-phenoxybenzylamino)acetate (18)
A mixture of glycine methyl ester hydrochloride (5.03 g; 40 mmol) in 25 mL dry methanol was treated with triethylamine (4.05 g; 40 mmol) and the reaction mixture was stirred for 10 minutes. A solution of benzaldehyde (4.24 g; 40 mmol) in dry methanol (12.5 mL) was added dropwise and the reaction mixture was stirred for 4 h at 0 °C under a nitrogen atmosphere. Sodium borohydride (3.03 g; 80 mmol) was added and the reaction mixture was stirred overnight at room temperature. The solvent was removed in vacuo, leaving a white solid which was washed with ether (3 × 10 mL) to give compound 18 (14.36 g; 100% yield). 1H NMR (CDCl3): δ 3.76 (s, 3H), 3.98 (s, 2H), 4.20 (s, 2H), 7.00–7.42 (m, 9H), 10.00 (s, 1H).
Methyl 2-((N-(tert-butoxycarbonyl)sulfamoyl)(3-phenoxybenzyl)amino)acetate (19)
A solution of t-butyl alcohol (2.43 g; 32.8 mmol) in 20 mL CH2Cl2 was added to a solution of chlorosulfonyl isocyanate (4.74 g; 32.8 mmol) in 20 mL CH2Cl2 at 0 °C. The resulting solution was added dropwise to a solution compound 18 (10.09 g; 32.8 mmol) in CH2Cl2 (80 mL) and the reaction mixture was stirred at room temperature overnight. The reaction mixture was washed with 5% HCl (100 mL), saturated NaHCO3 (100 mL), and brine (100 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent was removed, leaving a crude product which was purified by flash chromatography (silica gel/ethyl acetate/ hexanes) to give compound 19 as a white solid (6.30 g; 43% yield). 1H NMR (CDCl3): δ 1.50 (s, 9H), 3.76 (s, 3H), 4.02 (s, 2H), 4.60 (s, 2H), 6.90–7.18 (m, 6H), 7.21–7.39 (m, 4H).
Methyl 2-((3-phenoxybenzyl)(sulfamoyl)amino)acetate (20)
To compound 19 (10.9 g; 22.2 mmol) was added 60 mL trifluoroacetic acid and the reaction mixture was stirred at room temperature overnight. The trifluoroacetic acid was removed and the residue was taken up in ethyl acetate (100 mL) and washed with saturated sodium bicarbonate (3 × 50 mL) and brine (50 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and the solvent was removed to yield compound 20 as yellow oil (7.60 g; 99% yield). 1H NMR (CDCl3): δ 3.75 (s, 3H), 3.98 (s, 2H), 4.20 (s, 2H), 5.09 (s, 2H), 6.90–7.00 (m, 4H), 7.05–7.18 (m, 2H), 7.23–7.40 (m, 3H).
5-(3-Phenoxybenzyl)-1,2,5-thiadiazolidin-3-one 1,1-dioxide (21)
To a solution of compound 20 (7.6 g; 21.68 mmol) in dry THF (50 mL) cooled in an ice bath, was added sodium hydride (1.15 g; 60% w/w ; 28.8 mmol) in small portions and the reaction mixture was stirred at room temperature overnight. The solvent was removed, water (100 mL) was added water to the residue and the pH of the solution was adjusted to ~1. The aqueous layer was extracted with ethyl acetate (2 × 75 mL) and the organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent removed. The crude product was purified by flash chromatography (silica gel/ethyl acetate/ hexanes) to give compound 21 as yellow oil (6.75 g; 100% yield). 1H NMR (CDCl3): δ 3.82 (s, 2H), 4.37 (s, 2H), 4.90–5.20 (s, 1H), 6.92–7.18 (m, 6H), 7.22–7.40 (m, 3H). HRMS (ESI) calculated m/z C15H13N2O4S [M-H]+ 317.0596; found 317.0495.
Biochemical studies
One-day old, 80–90% confluent HG23 cells were treated with varying concentrations of each compound (0 [mock-DMSO]- 20 μM) to examine its effects on the replication of NV. At 24 or 48 hrs of treatment, the NV genome was analyzed with qRT-PCR. The ED50s of the compounds for NV genome levels were determined at 24 hr post-treatment. The cytotoxic effects of the compounds on HG23 cells were determined with varying concentrations of each compound (0 [mock-DMSO]-320 μM) using a cell cytotoxicity assay kit (Promega, Madison, WI) to calculate the median toxic dose (TD50) at 48 hr of treatment.
Acknowledgments
The generous financial support of this work by the National Institutes of Health (AI081891) is gratefully acknowledged.
References
- 1.Koo HL, Ajami N, Atmar RL, DuPont HL. Discov. Med. 2010;10:61. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao H. Drug Disc. Today. 2007;12:149. doi: 10.1016/j.drudis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Bohm H-J, Flohr A, Stahi M. Drug Discov. Today. 2004;1:217. doi: 10.1016/j.ddtec.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Kruse LI, Kaiser C, DeWolf WE, Finkelstein JA, Frazee JS, Hilbert EL, Ross ST, Flaim KE, Sawyer JL. J. Med. Chem. 1990;33:781. doi: 10.1021/jm00164a051. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Park HB, Lee JS, Jo NH, Yoo KH, Baek D, Kang B, Cho J-H, Oh C-H. Eur. J. Med. Chem. 2007;42:1176. doi: 10.1016/j.ejmech.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Burdinski D, Lub J, Pikkemaat JA, Moreno JD, Martial S, Del Pozo O. Dalton Transactions. 2008;31:4138. doi: 10.1039/b803557a. [DOI] [PubMed] [Google Scholar]
- 7.Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.(a) Shen J, Woodward R, Kedenburg JP, Liu X, Chen M, Fang L, Sun D, Wang PG. J. Med. Chem. 2008;51:7417. doi: 10.1021/jm8005355. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Demaray JA, Thuener JE, Dawson MN, Sucheck SJ. Bioorg. Med. Chem. Lett. 2008;18:4868. doi: 10.1016/j.bmcl.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 9.Njar CCO. Synthesis. 2000:2019. [Google Scholar]
- 10.Groutas WC, Kuang R, Venkataraman R, Epp JB, Ruan S, Prakash O. Biochemistry. 1997;37:4739. doi: 10.1021/bi9628937. [DOI] [PubMed] [Google Scholar]
- 11.Kuang R, Epp JB, Ruan S, Chong LS, Venkataraman R, Tu J, He S, Truong TM, Groutas WC. Bioorg Med Chem. 2000;8:1005. doi: 10.1016/s0968-0896(00)00038-9. [DOI] [PubMed] [Google Scholar]
- 12.Lai Z, Gan X, Wei L, Alliston KR, Yu H, Li YH, Groutas WC. Arch Biochem Biophys. 2004;429:191. doi: 10.1016/j.abb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. Virology. 2006;2:463. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Chang KO, George DW. J. Virol. 2007;22:12111. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang KO. J. Virol. 2009;83:8587. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan M, Jiang X. Curr. Opin. Investig. Drugs. 2008;9:146. [PubMed] [Google Scholar]
- 17.Guiard J, Fiege B, Kitov PI, Peters T, Bundle DR. Chem. Eur. J. 2011;17:7438. doi: 10.1002/chem.201003414. [DOI] [PubMed] [Google Scholar]
- 18.Rademacher C, Guiard J, Kitov PI, Fiege B, Dalton KP, Parra F, Bundle DR, Peters T. Chem. Eur. J. 2011;17:7442. doi: 10.1002/chem.201003432. [DOI] [PubMed] [Google Scholar]
- 19.Rishton GM. Drug Discov. Today. 2003;8:86. doi: 10.1016/s1359644602025722. [DOI] [PubMed] [Google Scholar]