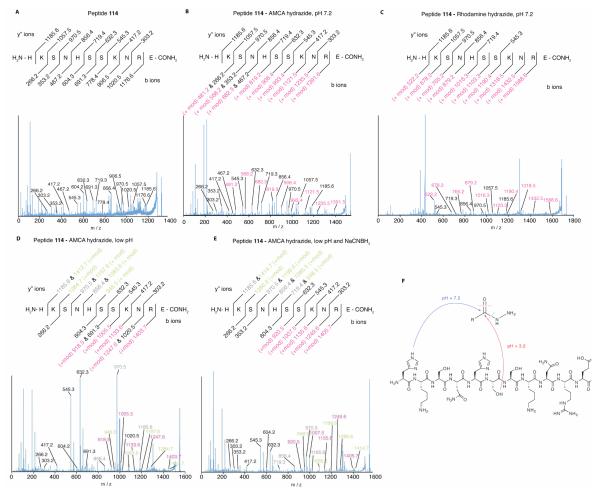
Figure 6.
MSMS of products from the reaction of peptide 114 with AMCA and rhodamine B hydrazides. (A) MSMS of unmodified peptide 114 reveals all expected b and y” ions. (B) and (C) MSMS of both the AMCA- and rhodamine-modified peptides reveals an unmodified set of y” ions and a complete set of modified b ions indicating the covalent modification (+mod) occurs on the side chain of the N-terminal histidine. (D) MSMS of the reaction product at low pH and (E) following reduction with NaCNBH3 product indicates that the covalent modification occurs on Ser6. The MSMS chromatogram of peptide 114 modified with rhodamine B hydrazide at low pH was dominated by rhodamine cleavage adducts (data not shown). (F) A schematic diagram for the observed covalent modifications.