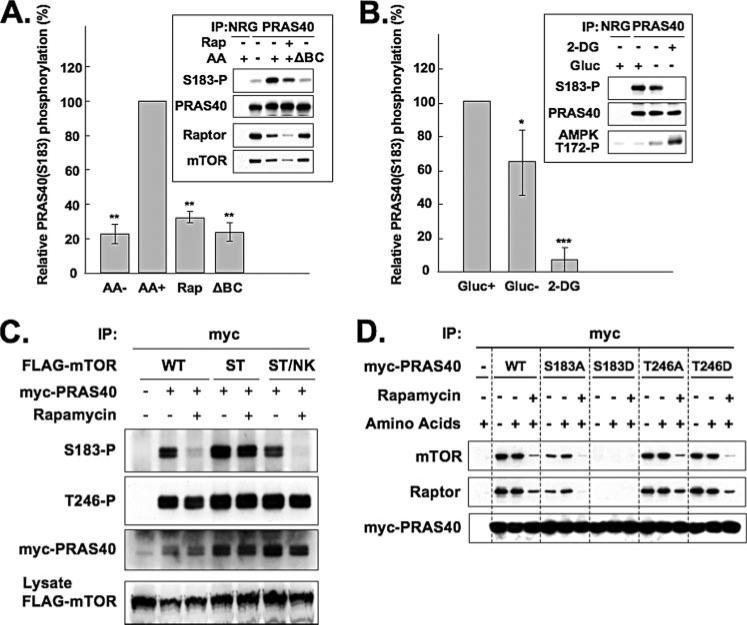
FIGURE 5. Phosphorylation of PRAS40 at Ser183 in vivo.
A, effects of amino acids and rapamycin on phosphorylation of PRAS40 at Ser183. HEK293 cells were deprived of serum, further incubated without amino acids, and stimulated by the readdition of amino acids (AA) in the presence or absence of rapamycin (Rap). Where indicated, the amino acid mixture readded lacked branched-chain amino acids (ΔBC). The cell lysates were subjected to immunoprecipitation with the anti-PRAS40 antibody. The immunoprecipitate by the NRG was employed as a control. Immunoblot was carried out with the anti-PRAS40 (Ser(P)183), anti-PRAS40, anti-raptor, and anti-mTOR antibodies, and the blots were scanned. A representative experiment is shown in the inset. The OD of the PRAS40 (Ser(P)183) blot was divided by the OD of the corresponding PRAS40 blot. This ratio for the AA+ condition was set to 100% and divided into the ratio for each of the other conditions. The bar graphs summarize the results of three experiments. The double asterisks indicate a reduction as compared with AA+, p < 0.01. B, effect of ATP depletion on phosphorylation of PRAS40 at Ser183. HEK293 cells were deprived of serum and incubated in the absence of glucose (Gluc) alone or with added 2-deoxyglucose (2-DG). The cell lysates were subjected to immunoprecipitation with the anti-PRAS40 antibody. The immunoprecipitate by the NRG was employed as a control. Immunoblot was carried out with the anti-PRAS40 (Ser(P)183), anti-PRAS40, and anti-raptor antibodies. The aliquots of the cell lysates were subjected to immunoblot by the anti-AMPK (Thr(P)172) antibody. A representative experiment is shown in the insets. The OD of the PRAS40 (Ser(P)183) blot was divided by the OD of the corresponding PRAS40 blot. This ratio for the Gluc+ condition was set to 100% and divided into the ratio for each of the other conditions. The bar graphs summarize the results of four experiments. The double or single asterisks indicate a reduction as compared with Gluc+; p < 0.01 or p < 0.05, respectively. C, an active, rapamycin-resistant mTOR mutant overcomes rapamycin-induced dephosphorylation of PRAS40 (Ser183) in vivo. HEK293 cells co-transfected with Myc-tagged PRAS40 and either FLAG-tagged wild-type mTOR (WT), rapamycin-resistant mTOR (S2035T) mutant (ST), or rapamycin-resistant/kinase-negative mTOR (S2035T/N2343K) mutant (ST/NK) were deprived of serum, further incubated without amino acids, and stimulated by the readdition of amino acids in the presence or absence of rapamycin. The cell lysates were subjected to immunoprecipitation with the anti-Myc antibody. Immunoblot was carried out with the anti-PRAS40 (Ser(P)183), anti-PRAS40 (Thr(P)246), and anti-Myc antibodies. The aliquots of the cell lysates were subjected to immunoblot by the anti-FLAG antibody. D, mutation of PRAS40 (Ser183) to Asp abolishes association with raptor. HEK293 cells transfected with Myc-tagged PRAS40, its mutants, or the empty vector were deprived of serum, further incubated without amino acids, and stimulated by the readdition of amino acids in the presence or absence of rapamycin. The cell lysates were subjected to immunoprecipitation with the anti-Myc antibody. Immunoblot was carried out with the anti-mTOR, anti-raptor, and anti-Myc antibodies.