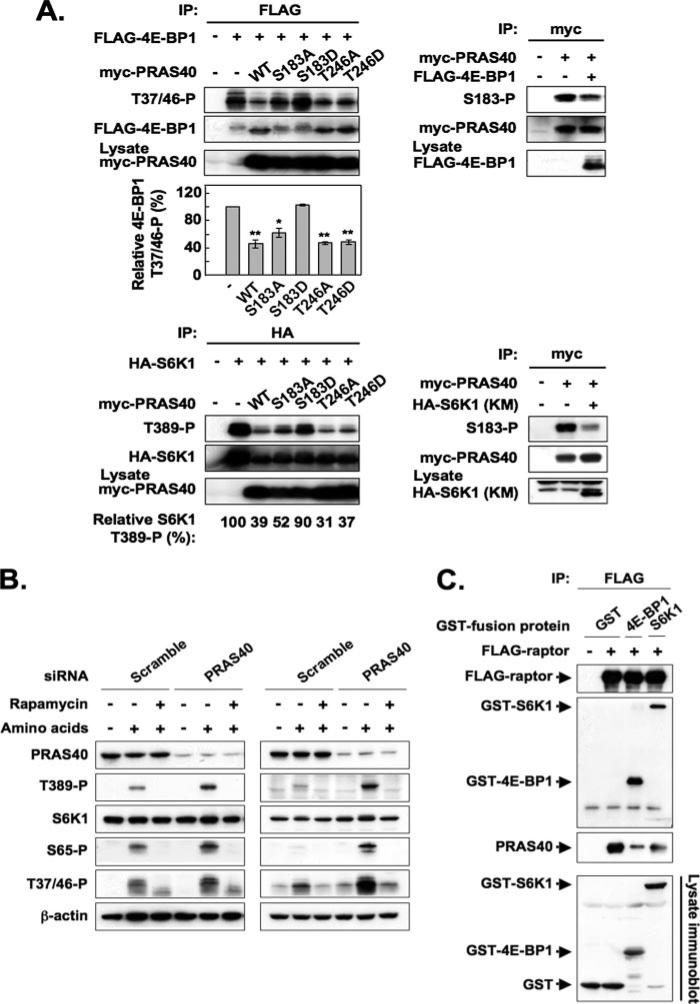
FIGURE 7. Competitive effects among mTORC1 substrates for raptor binding and mTORC1 phosphorylation in vivo.
A, effect of overexpression of an mTORC1 substrate on phosphorylation of the other two substrates. In the experiments shown in the four panels on the upper left, HEK293 cells were co-transfected with FLAG-tagged 4E-BP1 and either empty vector, Myc-tagged PRAS40 wild type, or the PRAS40 mutants indicated. The cells were deprived of serum, further incubated without amino acids, and stimulated by the readdition of amino acids. The aliquots of the cell lysates were subjected to immunoblot by the anti-Myc antibody to verify comparable expression of the PRAS40 variants. An anti-FLAG immunoprecipitate was immunoblotted for 4E-BP1 (Thr(P)37/46) and FLAG. The upper three panels show the immunoblots from a representative experiment. The 4E-BP1 (Thr(P)37/46) blots were scanned, and the OD was divided by the OD of the corresponding FLAG-4E-BP1 blot. This ratio for the FLAG immunoprecipitate from cells that did not receive Myc-PRAS40 (vector only) was set to 100% and divided into the ratio obtained for each of the other FLAG immunoprecipitates. The bar graph summarizes the results from three experiments. The double or single asterisks indicate a reduction as compared with vector only; p < 0.01 or p <0.05, respectively. In the three panels shown at the lower left, HA-tagged S6K1 was co-expressed with either empty vector, Myc-tagged PRAS40 wild type, or the PRAS40 mutants indicated. The aliquots of the cell lysates were subjected to immunoblot by the anti-Myc antibody to verify comparable expression of the PRAS40 variants. An anti-HA immunoprecipitate was immunoblotted for S6K1 (Thr(P)389) and HA, and the blots were scanned. The OD of the S6K1 (Thr(P)389) blot was divided by the OD of the corresponding HA-S6K1 blot. This ratio for the HA immunoprecipitate from cells that did not receive Myc-PRAS40 (vectoronly) was set to 100% and divided into the ratio obtained for each of the other HA immunoprecipitates. These values of one experiment are shown below the bottom panel; a second experiment gave similar results. The panels on the right show the effects of 4E-BP1 (upper) and kinase-negative S6K1 (lower) co-expression on PRAS40 (Ser183) phosphorylation; cells were treated in a manner similar to the experiments on the left. B, effect of RNA interference-induced PRAS40 depletion on the phosphorylation of endogenous S6K1 and 4E-BP1. HEK293 cells (left) and HeLa cells (right) transfected with indicated siRNA were deprived of serum, further incubated without amino acids, and stimulated by the readdition of amino acids in the presence or absence of rapamycin. The cell lysates were subjected to immunoblot by the anti-PRAS40, anti-S6K1 (Thr(P)389), anti-S6K1, anti-4E-BP1 (Ser(P)65), anti-4E-BP1 (Thr(P)37/46), and anti-β-actin antibodies. C, effect of overexpression of 4E-BP1 and S6K1 on the association of endogenous PRAS40 with overexpressed recombinant raptor. HEK293 cells co-transfected with FLAG-tagged raptor and either GST, GST-fused 4E-BP1, or GST-fused S6K1 were deprived of serum and further incubated without amino acids. The cell lysates were subjected to immunoprecipitation with the anti-FLAG antibody. Immunoblot was carried out with the anti-FLAG, anti-GST, and anti-PRAS40 antibodies. The aliquots of the cell lysates were subjected to immunoblot by the anti-GST antibody.