Abstract
AMPAR (α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid receptor) is an ion channel involved in the formation of synaptic plasticity. However, the molecular mechanism that couples plasticity stimuli to the trafficking of postsynaptic AMPAR remains poorly understood. Here, we show that PIKE (phosphoinositide 3-kinase enhancer) GTPases regulate neuronal AMPAR activity by promoting GluA2/GRIP1 association. PIKE-L directly interacts with both GluA2 and GRIP1 and forms a tertiary complex upon glycine-induced NMDA receptor activation. PIKE-L is also essential for glycine-induced GluA2-associated PI3K activation. Genetic ablation of PIKE (PIKE−/−) in neurons suppresses GluA2-associated PI3K activation, therefore inhibiting the subsequent surface expression of GluA2 and the formation of long-term potentiation. Our findings suggest that PIKE-L is a critical factor in controlling synaptic AMPAR insertion.
Keywords: GluA2, GRIP1, LTP, PIKE, PI3K
Introduction
PIKE (phosphoinositide 3-kinase enhancer) is a family of GTPases that directly interact and enhance the activity of phosphoinositide 3-kinase (PI3K) and Akt (Ye et al, 2000; Rong et al, 2003; Ahn et al, 2004). Through different promoters and alternative splicing of CENTG1 gene, three PIKE isoforms (PIKE-L, PIKE-S and PIKE-A) are generated (Chan and Ye, 2007). Our previous studies showed that PIKE promote cell survival, which functionally links extracellular stimuli to the PI3K/Akt pathway. For instance, PIKE-L interacts with Homer-1 and metabotropic glutamate receptor I (mGlu1) during mGlu1 activation in hippocampal neurons, leading to the activation of PI3K and prevention of neuronal apoptosis (Rong et al, 2003). It also associates with netrin-1 receptor Unc5B to activate PI3K upon netrin stimulation, thus protecting neurons from apoptosis (Tang et al, 2008). Recently, we reported that PIKE-L partnered with the DNase inhibitor SET, and prevented it from cleavage by endopeptidase AEP during excitotoxicity and stroke (Liu et al, 2008). Thus, PIKE-L is critical in maintaining neuronal survival against apoptotic stimuli.
Long-term potentiation (LTP) is a cellular model of memory formation and consolidation, resulting from long-lasting alternations in the efficacy of synaptic transmission (Bliss and Collingridge, 1993). Accumulating evidences suggest that trafficking of postsynaptic α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid receptor (AMPAR) is critical for synaptic plasticity (Genoux and Montgomery, 2007). AMPAR is an ionotropic glutamate receptor that contributes the majority of fast excitatory synaptic transmission (Palmer et al, 2005). It is a tetrameric ion channel composed of various combinations of four subunits (GluA1 to GluA4) and gates the passage of Na+, K+ and Ca2+ ions (Rosenmund et al, 1998). While incorporation of GluA2 to AMPAR renders the passage of Ca2+, AMPAR with Q/R unedited GluA2 is calcium permeable (Burnashev et al, 1992). Although many mechanisms like structural remodelling of synapse and alternation in gene transcription could lead to the change of synaptic plasticity, it is in consensus that addition of AMPAR to postsynaptic surface mediates the formation of LTP (Muller et al, 2000; Costa-Mattioli et al, 2009; Santos et al, 2009).
Trafficking of AMPAR to the synaptic membrane is a dynamic process that occurs constitutively between the cytoplasm and cell surface, and is facilitated by several proteins. For example, export of AMPAR from the ER to cell surface is controlled by molecular chaperones TARP (transmembrane AMPAR regulatory proteins) (Ziff, 2007). It has also been reported that PICK1 (protein interacting with C-kinase 1) was required for AMPAR’s endocytosis (Xia et al, 1999; Lu and Ziff, 2005). In addition, it is suggested that GRIP1 (glutamate receptor-interacting protein 1), a seven PDZ-containing protein that interacts with the C-terminus of GluA2, is essential for AMPAR trafficking (Dong et al, 1997; Srivastava et al, 1998; Matsuda et al, 1999; Wyszynski et al, 1999; Osten et al, 2000; Zhang et al, 2001). Functioning as an adaptor protein, GRIP1 links AMPAR with microtubule motor proteins kinesins to facilitate the receptor transportation along the cell body (Setou et al, 2002; Hoogenraad et al, 2005). Synaptic targeting of AMPAR could also be regulated during synaptic plasticity (Song and Huganir, 2002). Pioneer studies by electrophysiological method in the CA1 region of hippocampus suggest that the synaptic level of AMPAR is rapidly enhanced during tetanic stimulation (Lissin et al, 1998). This activity-induced delivery of AMPAR requires synaptic N-methyl-D-aspartic acid receptor (NMDAR) activation (Shi et al, 1999). Indeed, activation of NMDAR by either tetanic stimulation or partial agonist glycine is sufficient to induce postsynaptic AMPAR insertion (Lu et al, 2001). Studies by Man et al (2003) further reveal that the glycine-induced AMPAR trafficking depends on the GluA2-associated PI3K activity. In this model, Ca2+ influx through the activated NMDAR results in an activation of GluA2-associated PI3K and accumulation of membrane binding phosphoinositide-3,4,5-trisphosphate (PtdIns-3P), which in turn, facilitates membrane targeting of AMPAR. However, how PtdIns-3P triggers the delivery of AMPAR from its intracellular pool to cell surface remains unknown.
Here, we report that PIKE-L acts as a novel interacting partner to both GRIP1 and GluA2, which enhances the formation of GluA2/GRIP1 complex during glycine-induced LTP. Moreover, PIKE-L is critical for glycine to trigger GluA2-associated PI3K activation. Depletion of PIKE in PIKE knockout (PIKE−/−) mice changes the AMPAR activity and abolishes the glycine-induced AMPAR-associated PI3K activation, thus inhibiting the subsequent GluA2 trafficking and LTP formation.
Results
PIKE interacts with GRIP1
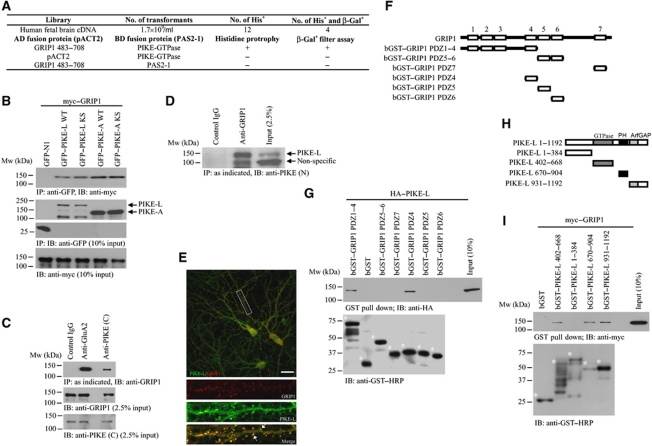
To look for the binding targets of PIKE-L, we performed a yeast two-hybrid (Y2H) analysis using the GTPase domain as the bait. Four of the 12 independent positive clones were identified as overlapping fragments of GRIP1 protein. We observed the interaction between the GTPase domain of PIKE-L and PDZ domain 4–6 (amino acids 483–708) in GRIP1 regardless of which protein was used as bait or prey (Figure 1A). To verify the interaction between these two proteins in a cellular context, we conducted the binding assay in HEK293 cells. Both wild-type (WT) and GTPase dominant-negative (KS) PIKE-L interacted strongly with GRIP1 (Figure 1B). Similarly, both PIKE-A WT and PIKE-A KS associated with GRIP1 as well, indicating that the N-terminal domain (NTD) and GTPase activity of PIKE are dispensable for their associations with GRIP1. The interaction of PIKE-L and GRIP1 was readily detectable in cultured neurons or brain tissues (Figure 1C, fourth lane, and Figure 1D, second lane). The assay specificity was confirmed by the negative signal in the control IgG (Figure 1C, first lane, and Figure 1D, first lane). Immunofluorescence staining on primary hippocampal neurons revealed the co-localization (Rr=0.58±0.03, n=3) of PIKE-L and GRIP1 in dendrites (Figure 1E), which further supported the physiological interaction of these two proteins. This staining was specific as the signal was blocked by antigen used in generating PIKE-L antibody but not by GST control protein (data not shown).
Figure 1.
PIKE associates with GRIP1. (A) Y2H screening using PIKE-L GTPase domain as the bait. (B) Both PIKE-A and PIKE-L interact with GRIP1. HEK293 cells were co-transfected with myc–GRIP1 and various GFP-tagged PIKE-L or PIKE-A constructs. PIKE proteins were immunoprecipitated and the associated GRIP1 was detected (first panel). The expression of GFP proteins (second and third panels) and myc–GRIP1 was also examined (fourth panel). (C) PIKE-L associates with GRIP1 in neurons. Immunoprecipitation using various antibodies was performed as indicated in cultured cortical neurons (21 DIV) and the associated proteins were detected using specific antibody (top panel). Total GRIP1 (middle panel) was detected using antibody against GRIP1. Antibody against the C-terminus of PIKE-L (PIKE (C)) was used to determine the expression of PIKE-L (bottom panel). (D) PIKE-L associates with GRIP1 in rat brain. Immunoprecipitation using indicated antibody was performed in whole brain lysates and the associated PIKE-L was detected by antibody against the N-terminus of PIKE-L (PIKE (N)). (E) Co-localization of PIKE-L and GRIP1 in neurons. Immunofluorescent staining was performed on hippocampal neurons (21 DIV) using anti-PIKE-L C-terminus antibody (PIKE (C)) (green) and anti-GRIP1 antibody (red). Scale bar represents 20 μm. (F) Schematic representation of various GRIP1 deletion truncates used in the in vitro binding assay. (G) Mapping of PIKE-L interaction domain in GRIP1. Various deletion truncates of GRIP1 tagged with bacterial GST were purified and incubated with cell lysates from HEK293 cells expressing HA–PIKE-L. The GST proteins were pulled down and the associated PIKE-L was detected (upper panel). The expression of GST-tagged GRIP1 truncates (asterisked) was also examined (lower panel). (H) Schematic representation of various PIKE-L deletion truncates used in the in vitro binding assay. (I) Mapping of GRIP1 interaction domain in PIKE-L. Various deletion mutants of PIKE-L were expressed and purified from bacteria and incubated with cell lysates from HEK293 cells expressing myc–GRIP1. The GST proteins were pulled down and the associated PIKE-L was detected (upper panel). The expression of GST-tagged PIKE-L truncates (asterisked) was also examined (lower panel).
To delineate the PIKE-L-binding site in GRIP1, we performed an in vitro mapping assay. PIKE-L strongly bound to PDZ4 but not other PDZ domains (Figure 1G; Supplementary Figure S1B). To identify the domain in PIKE-L responsible for the PIKE/GRIP1 interaction, we conducted co-immunoprecipitation assay after co-transfecting various deletion mutants of GFP-tagged PIKE-L and myc–GRIP1 into HEK293 cells (Figure 1H). While the GTPase, PH and the ArfGAP domains associated with GRIP1, the proline-rich NTD failed (Figure 1I), suggests that the NTD in PIKE-L is dispensable for the PIKE-L/GRIP interaction.
PIKE-L associates with AMPAR
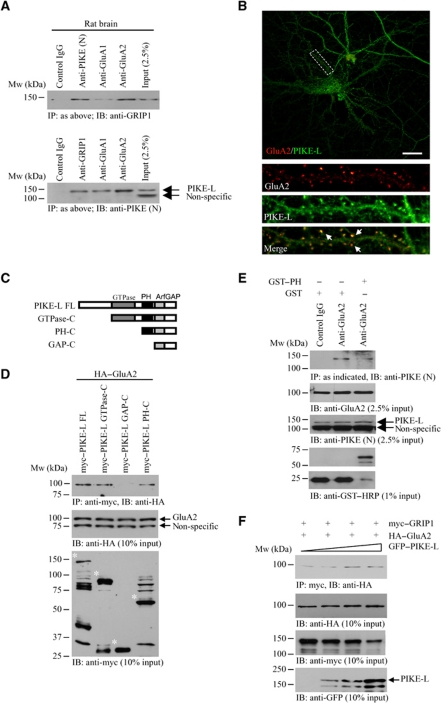
Given that GRIP1 is a binding partner of AMPAR (Dong et al, 1997), and PIKE-L interacts with other glutamate receptor family member (Rong et al, 2003), we speculated that PIKE-L, GRIP1 and GluA2 might exist as a functional complex. To test this hypothesis, we performed co-immunoprecipitation in rat brain tissue. A PIKE-L/GRIP1 complex was readily detected no matter anti-PIKE-L or anti-GRIP1 was employed in the immunoprecipitation (Figure 2A, upper and lower panels, second lane). PIKE-L was also co-immunoprecipitated with GluA2 (Figure 2A, lower panel, fourth lane). Since the majority of AMPARs in brain are GluA1/GluA2 heterodimers (Sans et al, 2003), it is reasonable to find that both PIKE-L and GRIP1 were pulled down when anti-GluA1 was utilized (Figure 2A, upper and lower panels, third lane). This was supported by the fact that PIKE-L associated specifically with GluA2 but not GluA1 in HEK293 cells (Supplementary Figure S2A). Similarly, we did not observe any significant binding between PIKE-L and GluA3 in mouse brain lysate (data not shown). These results strongly suggest that PIKE-L, GRIP1 and AMPAR form a complex in brain. Immunofluorescent staining further demonstrated the co-localization (Rr=0.50±0.05, n=3) of PIKE-L and GluA2 in dendrites of cultured hippocampal neurons (Figure 2B).
Figure 2.
PIKE associates with GluA2. (A) Formation of PIKE-L/GRIP1/GluA2 complex in brain. Immunoprecipitation from whole rat brain lysates using various antibodies was performed and detected with either anti-GRIP1 antibody or antibody against the N-terminus of PIKE-L (PIKE (N)). (B) Co-localization of PIKE-L and GluA2 in neurons. Immunofluorescent staining was performed in hippocampal neurons (21 DIV) using anti-PIKE-L C-terminus antibody (PIKE (C)) (red) and anti-GluA2 antibody (green). Scale bar represents 20 μm. (C) Schematic representation of various PIKE-L deletion truncates used in the in vitro binding assay. (D) The PH domain of PIKE-L is essential for GluA2 interaction. HEK293 cells were transfected with HA–GluA2 and different deletion mutant of myc–PIKE-L. The PIKE-L truncates were immunoprecipitated and the associated GluA2 was detected (top panel). The expression of the GluA2 (middle panel) and PIKE-L (asterisked) (bottom panel) was also examined. (E) Inhibition of PIKE-L/GluA2 interaction by exogenous PIKE-L PH domain. Mouse brain lysates were first incubated with 50 μg recombinant GST protein or GST–PIKE-L PH domain (GST–PH) and the PIKE-L were then immunoprecipitated. The associated GluA2 was then detected (first panel). The expressions of GluA2 (second panel), PIKE-L (third panel) and the GST proteins (fourth and fifth panels) were also examined. (F) PIKE-L enhances GRIP1/GluA2 interaction. HEK293 cells were transfected with HA–GluA2, myc–GRIP1 and various amount of GFP–PIKE-L. Interaction of GRIP1 and GluA2 was detected using immunoprecipitation (first panel). The expression of GluA2 (second panel), GRIP1 (third panel) and PIKE-L (fourth panel) was also examined (bottom panel).
To determine the GluA2-interacting motif in PIKE-L, we performed an in vitro mapping assay. The GluA2/PIKE-L interaction in 293 cells was abolished when the PIKE-L PH domain was deleted (Figure 2D), indicating that the PH domain is essential for the interaction. To further demonstrate that the PIKE-L associates with GluR2 through its PH domain, we introduced recombinant GST-tagged PH domain (GST–PH) or GST proteins into the mouse brain lysates. Preincubation with GST–PH but not GST protein alone diminished the association between PIKE-L and GluA2, suggesting that PIKE-L PH domain is necessary for mediating GluA2 binding (Figure 2E).
We have also examined the PIKE-L-binding motif in GluA2. WT, PDZ-binding site mutated (myc–R2 SVKE) or C-terminal truncated (myc–R2 1–853) GluA2 (Supplementary Figure S2B) were co-transfected with GFP–PIKE-L into HEK293 cells, followed by co-immunoprecipitation. WT GluA2 and all of the mutants strongly associated with PIKE-L (Supplementary Figure S2C), suggesting that PIKE-L bound to the juxtapose region in the GluA2 C-terminal tail.
Next, we tested if the association of PIKE-L and GluA2 depends on GRIP1. In HEK293 cells, PIKE-L interacted with GluA2 in the absence of GRIP1. The association of PIKE-L and GluA2 was not increased and even the expression of GRIP1 was quadruplicated (Supplementary Figure S2D). Therefore, GRIP1 is dispensable for PIKE-L/GluA2 association. However, the association of GluA2 and GIRP1 was augmented in the presence of PIKE-L in a dose-dependent manner (Figure 2F, first panel), indicating that PIKE-L enhanced the binding of GRIP1 to GluA2.
PIKE-L is essential for glycine-induced GluA2-associated PI3K activation and GluA2 surface expression
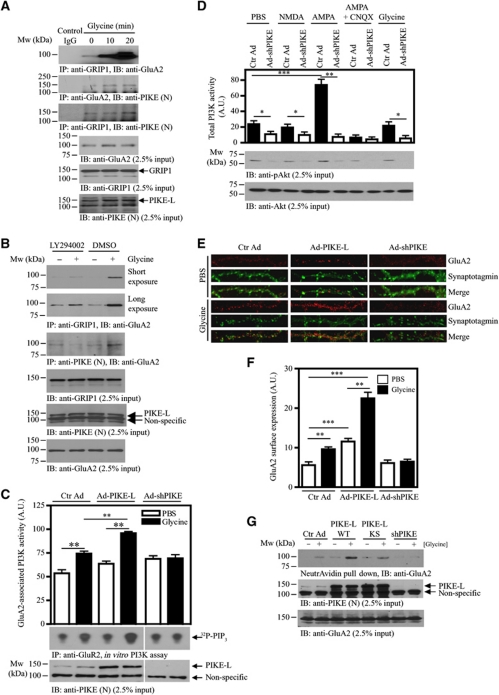
Glycine is an obligatory co-agonist of glutamate to fully activate NMDAR (Johnson and Ascher, 1987). It has been reported that glycine application to cultured hippocampal neurons elicited LTP by increasing postsynaptic AMPAR number (Lu et al, 2001). Since GRIP1 is a critical protein in facilitating GluA2 surface expression (Osten et al, 2000), we hypothesized that glycine might induce GluA2/GRIP1 association. Primary neurons were treated with glycine (200 μM) for 3 min followed by a recover period of 10 or 20 min (Man et al, 2003). In these neurons, the association of GRIP1 with GluA2 increased strongly after glycine induction in a time-dependent manner (Figure 3A, first panel). Concomitantly, PIKE-L/GluA2 interaction also increased (Figure 3A, second panel), suggesting that the formation of PIKE-L/GluA2 complex was enhanced upon glycine stimulation. On the other hand, PIKE-L constitutively associated with GRIP1, and their interaction increased slightly after 20 min stimulation of glycine (Figure 3A, third panel). The association of GluA2/GRIP1 or GluA2/PIKE-L was PI3K dependent as inhibition of PI3K by LY294002 abrogated the glycine-induced interactions (Figure 3B, first and third panels). Since GluA2 constitutively associates with PI3K that could be activated by glycine (Man et al, 2003), presumably, activation of GluA2-associated PI3K (PI3KGluA2) may serve as the molecular switch to recruit PIKE-L and GRIP1 to GluA2.
Figure 3.
PIKE is essential for glycine-induced GluA2-associated PI3K activation and GluA2 surface expression in neurons. (A) Glycine stimulation enhances GluA2/GRIP1 association. Cortical neurons (10 DIV) were treated with glycine (200 μM) for indicated time intervals and the interactions between GRIP1, GluA2 and GRIP1 were determined by immunoprecipitation (first to third panels). The expressions of GluA2 (fourth panel), GRIP1 (fifth panel) and PIKE-L (anti-PIKE-L N-terminus antibody) (sixth panel) under glycine stimulation were also verified. (B) PI3K is critical for GluA2/GRIP1 interaction. Cortical neurons (10 DIV) were pretreated with LY294002 (10 μM) for 1 h followed by 20 min glycine (200 μM) stimulation. The interaction between GRIP1, GluA2 and PIKE-L was determined by immunoprecipitation (first and third panels). PIKE-L level was examined using anti-PIKE-L N-terminus antibody (PIKE (N)) (fifth panel). The expressions of GRIP1 (third panel) and GluA2 (sixth panel) was also verified. (C) PIKE-L facilitates glycine-induced GluA2-associated PI3K activation. Cortical neurons (10 DIV) were infected with control adenovirus (Ctr Ad), adenovirus carrying PIKE-L (Ad-PIKE-L) or adenovirus carrying shRNA against PIKE-L (Ad-shPIKE). After 48 h infections, the neurons were treated with glycine (200 μM) and GluA2 was then immunoprecipitated. The activity of PI3K associated with GluA2 was examined by in vitro PI3K assay. Quantitation of 32P-PIP3 (middle panel) as a representation of PI3K activity was shown (top panel) and the expression of PIKE-L under various adenovirus infections was examined (bottom panel). Results were expressed as mean±s.e.m. (**P<0.01, Student's t-test, n=3). (D) Total PI3K activity could not be enhanced by glycine stimulation. Cortical neurons (10 DIV) were infected with either control adenovirus (Ctr Ad) or adenovirus carrying shRNA against PIKE-L (Ad-shPIKE). After 48 h infection, the neurons were treated with glycine (200 μM) and the total PI3K was then immunoprecipitated and analysed directly by in vitro PI3K assay (top panel). Phosphorylation of Akt was also examined using immunoblotting (middle panel). Total Akt was checked to ensure equal loading (bottom panel) (*P<0.05; **P<0.01; ***P<0.001, Student's t-test, n=3). (E) Overexpression of PIKE-L facilitates glycine-induced cell-surface expression of GluA2 in neurons. Hippocampal neurons (21 DIV) were infected with control adenovirus (Ctr Ad, left panels), adenovirus carrying PIKE-L (Ad-PIKE-L, middle panels) or adenovirus carrying shRNA against PIKE-L (Ad-shPIKE, right panels). After 48 h infection, the neurons were treated with glycine (200 μM), stained with anti-GluA2 antibody under non-permeabilized condition and the cell surface GluA2 were visualized by confocal microscope. (F) Quantitation of cell surface GluA2 on neurons shown in (D) (**P<0.01, ***P<0.001, Student's t-test, n=8). Arbitrary units (A.U.) of GluA2 expression were shown. (G) Biotinylation assay on cell-surface expression of GluA2. Cortical neurons (DIV 10) were infected with control adenovirus (Ctr Ad), adenovirus carrying PIKE-L (Ad-PIKE-L) or adenovirus carrying shRNA against PIKE-L (Ad-shPIKE) for 48 h before glycine (200 μM) treatment. Cell-surface proteins were biotinylated and pulled down by NeutrAvidin beads and the amount of GluA2 was detected using immunoblotting analysis (top panel). The expression of PIKE-L (middle panel) was determined using anti-PIKE-L N-terminus antibody (PIKE (N)). GluA2 level (bottom panel) after adenovirus infection was also examined.
PIKE-L coupled mGlu1 to PI3K pathway through binding its adaptor protein Homer (Rong et al, 2003), we thus hypothesized that PIKE-L might be necessary for glycine-induced PI3KGluA2 activation. In control hippocampal neurons, glycine treatment activated PI3KGluA2 (Figure 3C, lanes 1 and 2). Overexpression of WT PIKE-L further enhanced the activity of PI3KGluA2 after glycine stimulation (Figure 3C, lanes 3 and 4). On the other hand, depletion of PIKE-L by shRNA abolished the glycine-provoked PI3KGluA2 activity (Figure 3C, lanes 5 and 6), suggesting that PIKE-L was critical for the glycine-induced PI3KGluA2 activation. As expected, glycine did not elicit total cellular PI3K (PI3Ktotal) activation as compared with control (Figure 3D, lanes 1 and 9). This is in agreement with the previous finding that glycine-induced PI3K activation is unique to PI3KGluA2 but not the PI3Ktotal in cultured neurons (Man et al, 2003). Interestingly, PI3Ktotal activity was enhanced by AMPA stimulation and depletion of PIKE-L abolished this effect (Figure 3D, lanes 5 and 6), suggesting that PIKE-L was also important for the non-receptor-associated PI3K activation induced by AMPA. The AMPA-stimulated PI3K activation was abolished if the neurons were pretreated with AMPAR antagonist CNQX (Figure 3D, lanes 7 and 8). In contrast, NMDA stimulation did not increase the PI3Ktotal activity (Figure 3D, lanes 1 and 3). These results support that PIKE-L is also an important factor to regulate glutamate receptors-mediated PI3Ktotal activation.
Next, we sought to determine the effect of PIKE-L overexpression or depletion on GluA2 surface expression by synaptotagmin/GluA2 co-staining (Perestenko and Henley, 2003). Confocal microscopy analysis on hippocampal neurons indicated that the surface GluA2 expression induced by glycine treatment was greatly enhanced if PIKE-L was overexpressed (Figure 3E and F). In contrast, such increase in surface GluA2 expression was significantly reduced in PIKE-depleted neurons (Figure 3E and F; Supplementary Figure S3 for the whole morphology of the infected neurons). The change in GluA2 expression was further confirmed by biotinylation on cell-surface proteins (Man et al, 2007). In agreement with the immunofluorescence staining, the number of surface GluA2 was greatly elevated in primary neurons overexpressing PIKE-L WT (Figure 3G, lanes 2 and 4). PIKE-L KS expressing cells also had elevated surface GluA2 expression after glycine stimulation as compared with control, though they revealed less surface GluA2 than WT PIKE-L (Figure 3G, lanes 4 and 6), suggesting that the GTPase activity might not play a major role in this process. On the other hand, no increase in the surface GluA2 was observed in PIKE-depleted neurons, indicating that PIKE is critical for glycine-induced membrane GluA2 retention (Figure 3G, lanes 7 and 8).
Glycine-induced PI3K activity and GluA2 surface expression are abolished in PIKE−/− neurons
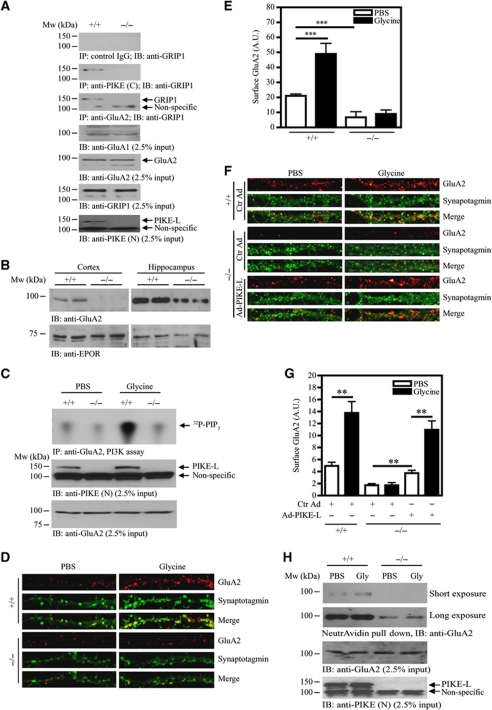
Next, we examined the effect of PIKE ablation on GluA2/GRIP1 interaction in PIKE knockout (PIKE−/−) mice (Supplemental data; Supplementary Figure S4). Association between GluA2 and GRIP1 was diminished in PIKE−/− brain (Figure 4A, third panel), suggesting that PIKE-L was critical for GRIP1/GluA2 interaction. Moreover, we have determined the amount of GluA2 on the cell surface of neurons in PIKE−/− brain. In the purified plasma membrane of neurons from cortex and hippocampus, expressions of GluA2 were diminished in samples isolated from PIKE−/− mice (Figure 4B). We also determined the effect of PIKE ablation on glycine-induced PI3KGluA2 activity. Glycine stimulation activated the PI3KGluA2 in cultured WT but not the PIKE−/− neurons (Figure 4C, top panel), which is in agreement with the result of shRNA knockdown assay (Figure 3C). Moreover, cell surface GluA2 insertion induced by glycine was also abolished in PIKE−/− neurons (Figure 4D and E; Supplementary Figure S5 for the whole neuron morphology). To further demonstrate the important role of PIKE-L in modulating GluA2 surface expression. We infected PIKE−/− neurons with adenovirus overexpressing PIKE-L to see if GluA2 expression could be rescued. As shown in Figure 4F and G (also see Supplementary Figure S6 for the whole neuron morphology), surface expression of GluA2 was significantly enhanced in PIKE-L-restored PIKE−/− neurons in both basal and glycine-stimulated conditions. The reduced surface GluA2 expression in PIKE−/− neurons was further supported by the results of cell-surface protein biotinylation assay (Figure 4H). Hence, PIKE is indispensable for glycine-induced AMPAR surface insertion and PI3KGluA2 activation.
Figure 4.
Glycine-induced cell-surface expression of GluA2 is impaired in PIKE−/− neurons. (A) The association of GluA2 and GRIP1 is reduced in PIKE−/− brain. Immunoprecipitations from WT (+/+) and PIKE knockout (−/−) mouse brain lysates using control IgG (first panel), anti-PIKE-L C-terminal antibody (PIKE (C)) (second panel) and anti-GluA2 (third panel) were performed and the associated GRIP1 was detected using anti-GRIP1 antibody. The expression of GluA1 (fourth panel), GluA2 (fifth panel) and GRIP1 (sixth panel). The level of PIKE-L (seventh panel) was also examined using anti-PIKE-L N-terminus (PIKE (N)) antibody. (B) Surface expression of GluA2 is reduced in PIKE−/− brain. Plasma membrane were isolated from the cortex and hippocampus of age-matched (3-month-old) WT (+/+) and PIKE−/− (−/−) mice. The expression of GluA2 was determined by immunoblotting analysis (upper panel). Expression of EPO receptor was also determined as a loading control (lower panel). (C) Glycine-induced GluA2-associated PI3K activity is abolished in PIKE−/− neurons. After treated with glycine (200 μM), the GluA2 in WT (+/+) and PIKE-knockout (−/−) cortical neurons (10 DIV) was immunoprecipitated for PI3K assay (top panel). The expression of PIKE-L (middle panel) and GluA2 (bottom panel) in the cortical neurons were verified using anti-PIKE-L N-terminus (PIKE (N)) antibody and anti-GluA2, respectively. (D) Cell-surface expression of GluA2 under glycine treatment is impaired in PIKE−/− neurons. Hippocampal neurons (21 DIV) were stained with anti-GluA2 antibody under non-permeabilized condition and the cell surface GluA2 were visualized by confocal microscope. (E) Quantitation of cell surface GluA2 on neurons shown in (C) (***P<0.001, Student's t-test, n=8). Arbitrary units (A.U.) of GluA2 expression were shown. (F) Cell-surface expression of GluA2 is rescued in PIKE−/− neurons after PIKE-L overexpression. Hippocampal neurons (21 DIV) were infected with control adenovirus (Ctr Ad) or adenovirus overexpressing PIKE-L (AD-PIKE-L) for 48 h. The cells were then stimulated with PBS or glycine (200 μM, 20 min) and stained with anti-GluA2 antibody under non-permeabilized condition. (G) Quantitation of cell surface GluA2 on neurons shown in (F) (**P<0.01, Student's t-test, n=8). Arbitrary units (A.U.) of GluA2 expression were shown. (H) Biotinylation assay on cell-surface expression of GluA2. Cortical neurons (DIV 10) from WT (+/+) and PIKE knockout (−/−) mice were treated with glycine (200 μM). Cell-surface proteins were biotinylated, pulled down by NeutrAvidin beads and the amount of GluA2 was detected using immunoblotting analysis (top panel). The expressions of GluA2 (middle panel) and PIKE-L (bottom panel) were also examined using anti-PIKE-L N-terminus (PIKE (N)) antibody and anti-GluA2, respectively.
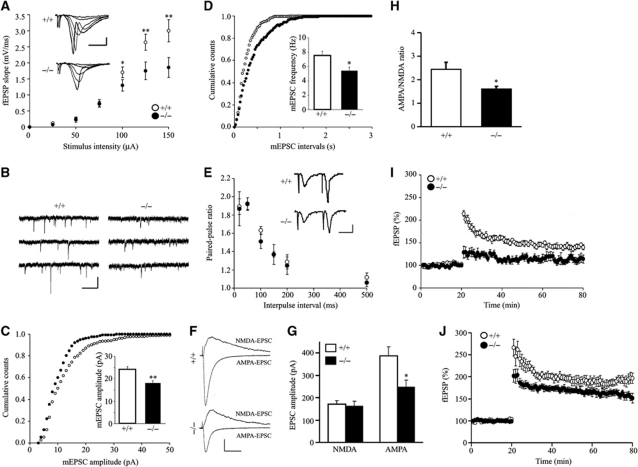
AMPA receptor-mediated transmission is impaired in the hippocampus of PIKE−/− mice
The observations that PIKE depletion reduces surface expression of GluA2 in cultured neurons suggest that AMPAR-dependent transmission in PIKE−/− mice may be impaired. To test this hypothesis, we first measured excitatory synaptic transmission at Schaffer Collateral-CA1 (SC-CA1) synapses by extracellular recording. In WT slices, field stimulus intensity correlated positively with the slope of field excitatory postsynaptic potentials (fEPSP) (Figure 5A). In contrast, the ratio of fEPSP slope to stimulus intensity (input–output ratio) of synaptic transmission was reduced in PIKE−/− hippocampal slices, indicating a defective synaptic transmission. To determine the underpinning cellular mechanisms, we recorded the miniature excitatory postsynaptic currents (mEPSCs) from CA1 neurons in the presence of 1 μM tetrodotoxin to block action potentials and 20 μM bicuculline to inhibit GABAergic transmission (Figure 5B). The mEPSCs were blockable by 10 μM AMPAR antagonist CNQX (data not shown). Remarkably, their amplitudes were significantly decreased in PIKE−/− CA1 neurons (Figure 5C). These results suggest that AMPAR density is reduced in CA1 neurons of PIKE−/− hippocampus, which is in agreement with the above biochemical and morphological studies.
Figure 5.
AMPA receptor-mediated synaptic transmission is impaired in PIKE−/− mice. (A) Reduced basal synaptic transmission in PIKE−/− hippocampus. The slopes of fEPSP were plotted against stimulus intensity. The insets show sample traces of fEPSPs at different stimulus intensity. Scale bars are 1 mV and 5 ms (nine slices from four WT mice (○) and seven slices from three PIKE−/− (•) mice, *P<0.05, **P<0.01 versus different genotypes under the same stimulation, Student's t-test). (B) Sample traces of mEPSC recording from CA1 neurons in whole-cell recording configuration. Scale bars are 20 pA and 100 ms. (C) Cumulative distribution for mEPSC amplitudes in WT (○) and PIKE−/− (•) neurons (WT, 24.2±1.3; PIKE−/−, 18.0±1.1 pA; n=8 cells from three WT and PIKE−/− mice, 4- to 6-week-old, **P<0.01, Student's t-test). (D) Cumulative distribution for mEPSC interevent intervals in WT (○) and PIKE−/− neurons (•) (WT, 7.6±0.5; PIKE−/−, 5.4±0.5 Hz; n=8 cells from three WT and PIKE−/− mice, 4- to 6-week-old, *P<0.05, Student's t-test). (E) Normal paired-pulse facilitation ratio in PIKE−/− SC-CA1 synapses. The insets show sample traces of fEPSPs at paired stimulation in 20 ms interval. Scale bars are 1 mV and 10 ms (nine slices from four WT mice (○) and seven slices from three PIKE−/− mice (•)). No statistically significant differences were detected in all conditions. (F) Representative overlays of NMDAR and AMPAR-evoked EPSCs from WT (top) and PIKE−/− (bottom) slices. NMDA-EPSC traces was obtained by subtracting the traces obtained in the presence of 100 μM DL-AP5 at +40 mV from those obtained before. The isolated AMPA-EPSC was measured at −60 mV in the presence of 100 μM DL-AP5. Scale bars represent 100 pA and 50 ms. (G) AMPA-EPSCs but not NMDA-EPSCs were reduced in PIKE−/− mice when compared with the WT mice (n=8 cells from three WT mice and n=9 cells from three PIKE−/− mice, *P<0.05, Student's t-test versus different genotypes). (H) AMPA/NMDA ratio (peak IAMPA/PEAK INMDA) is reduced in PIKE−/− mice (n=8 cells from three WT mice and n=9 cells from three PIKE−/− mice, *P<0.05, Student's t-test versus different genotypes). (I) TBS-induced LTP at SC-CA1 synapses was impaired in hippocampal slices of PIKE−/− mice (five slices from three WT mice (○) and six slices from three PIKE−/− mice (•)). (J) Reduced HFS-stimulated LTP in hippocampal slices of PIKE−/− mice. LTP at SC-CA1 synapses was induced by two trains HFS (five slices from three WT mice (○) and six slices from three PIKE−/− mice (•)).
The frequency of mEPSCs was also reduced in PIKE−/− CA1 neurons (Figure 5D). A decrease in mEPSC frequency usually reflects either a reduction in presynaptic transmitter release or a decrease in postsynaptic AMAP receptor. To address this question, we characterized paired-pulsed facilitation of fEPSPs and evoked EPSCs in response to two consecutive stimulations, which is a measurement of presynaptic function. The ratios were similar in PIKE−/− and WT slices in normal (Figure 5E) and GABA transmission-inhibited conditions (Supplementary Figure S7), suggesting that the probability of presynaptic release was not altered by PIKE deficiency. To test whether PIKE depletion caused any change in NMDAR-mediated transmission, we measured NMDAR-evoked EPSCs and compared the AMPA/NMDA ratio of evoked EPSC at SC-CA1 synapses using whole-cell recording. As shown in Figure 5F–H, NMDAR-mediated EPSCs were normal in PIKE−/− neurons but the AMPA/NMDA ratio was reduced, suggesting that AMPAR but not NMDAR-mediated transmission was impaired in PIKE−/− neurons.
We also studied hippocampal LTP induced by theta-burst stimulation (TBS) that resembled physiological hippocampal activity rhythm (Capocchi et al, 1992). As shown in Figure 5I, the slope of fEPSPs was increased for at least 60 min after induction in hippocampal slices from WT mice (142±5.1% of baseline), indicating the success of LTP induction. However, the same TBS failed to induce LTP in hippocampal slices of PIKE−/− mice (113±8.6% of baseline). We have also tested if LTP could be induced by a stronger stimulation in PIKE−/− brain slices. In contrast to TBS-induced LTP, two trains high frequency stimulation (HFS) (Opazo et al, 2003) was able to induce LTP in PIKE−/− neurons (Figure 5J). However, the LTP amplitude was smaller in PIKE−/− neurons (194±11.7% in WT versus 156±8.3% in PIKE−/−), suggesting that PIKE was an important modulator of LTP formation in a stimulation-dependent manner.
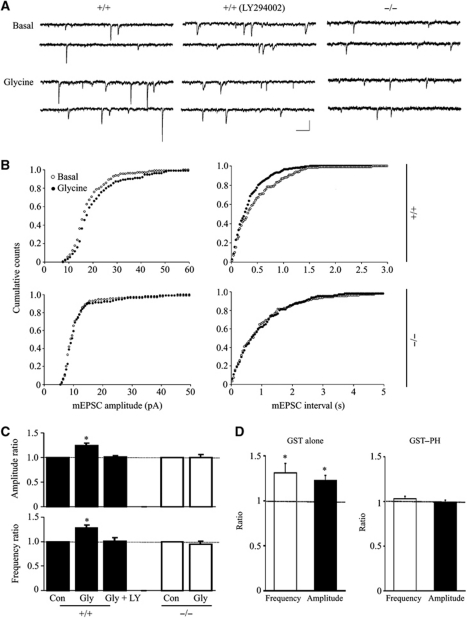
Glycine-induced LTP in PIKE−/− hippocampal neurons is compromised
Our biochemical and immunohistological studies indicate a critical role of PIKE-L in glycine-induced PI3KGluA2 activation and GluA2 surface expression. We thus investigated its role in glycine-induced LTP model of cultured hippocampal neurons. This form of LTP requires NMDAR and PI3K activation, which consequently increases plasma membrane insertion of AMPAR (Lu et al, 2001; Man et al, 2003). As shown in Figure 6, the amplitudes and frequencies of mEPSCs were increased in WT hippocampal neurons after stimulation with 200 μM glycine. The increased mEPSC frequency might be due to the increased number of detectable mEPSC, which could be below the detection threshold under basal condition. As reported previously (Man et al, 2003), inhibition of PI3K by LY294002 prevented the glycine effect (Figure 6A and C). In contrast, glycine was incapable of producing LTP in PIKE−/− neurons (mEPSC amplitude and frequency were similar to those of control) (Figure 6C), implying that PIKE deficiency impaired AMPA receptor-dependent LTP. We also infused GST–PH, the mutant that inhibits the GluA2/PIKE-L interaction (Figure 2E), into neurons through recording pipette and measured the change of mEPSC after glycine challenge. As a control, GST had no effect on glycine enhancement of mEPSCs. However, the enhancement was absent in cells with GST–PH domain in the patch pipette (Figure 6D). Together, our results demonstrate a critical role of the PIKE-L/GluA2 association in activity-dependent AMPAR insertion.
Figure 6.
Glycine-induced LTP induction is impaired in PIKE−/− hippocampal neurons. (A) Representative recordings from individual cultured neurons immediately before (Basal) and 20 min after glycine (200 μM) application. Middle traces were recorded from cultured hippocampal neurons that were treated with 10 μM LY294002 for 30 min prior to the application of glycine. Scale bars are 20 pA and 200 ms. (B) Cumulative distributions of mEPSC amplitudes and interevent intervals before (○) and 20 min after glycine (•). (C) Summary of data in (A, B). Responses obtained 20 min after glycine (Gly) treatment or LY294002 pretreatment (Ly) were normalized to the values from the initial 3 min (Con) of recording. Application of glycine in the bath solution induces LTP of mEPSC (1.24±0.05 and 1.28±0.06 for amplitude and frequency, respectively; n=8). Glycine produced LTP, which is prevented by inhibition of PI3K with LY294002 (1.01±0.03 and 1. 0±0.08 for amplitude and frequency, respectively; n=5) or in PIKE−/− cultured hippocampal neurons (0.99±0.06 and 0.95±0.06 for amplitude and frequency, respectively; n=6). Data were normalized by values before glycine application (*P<0.05, Student's t-test). (D) Inhibition of glycine-enhanced mEPSCs by PIKE-L PH domain. Neurons were recorded with pipette filled with solution containing 5 μg/ml GST (1.22±0.06 and 1.31±0.11 for amplitude and frequency, respectively; n=6) or GST-tagged PIKE-L PH domain (GST–PH; 1.03±0.03 and 0.99±0.02 for amplitude and frequency, respectively; n=5). Data were normalized by values before glycine application (*P<0.05, Student's t-test).
Discussion
The mechanism in controlling AMPAR trafficking is of great interest because of its importance in synaptic function and plasticity. Previous studies have suggested that expression of surface AMPAR depended on its association with various interacting proteins like NSF and GRIP1 (Dong et al, 1997; Nishimune et al, 1998). Here, we show that PIKE-L is a novel player that regulates AMPAR trafficking and LTP induction. By enhancing the GRIP1/GluA2 association during glycine-induced LTP induction, PIKE-L links the NMDA signal to AMPAR trafficking. Ablation of PIKE or disruption of PIKE-L/GluA2 interaction in neurons thus results in a reduction of GluA2 surface expression and LTP triggered by TBS or glycine challenge. Therefore, our results suggest that PIKE-L plays an important role in regulating AMPAR activity by controlling the surface expression of GluA2 subunit.
Several studies have shown that activation of PI3K modulates synaptic plasticity. However, little is known about the underlying mechanisms. PI3K inhibitors were shown to inhibit LTP expression, but not induction, in rat hippocampal CA1 (Sanna et al, 2002). However, in mouse hippocampal CA1, PI3K inhibition was reported to attenuate the induction, but not the expression (Opazo et al, 2003). Recent studies suggest that the effect of PI3K varies with different stimuli used to induce which LTP (Horwood et al, 2006; Peineau et al, 2007). It has also been reported that depletion of PtdIns-3P impaired LTP induction (Arendt et al, 2010). Moreover, PI3K is required for the induction of chemical-induced LTP (Man et al, 2003). Data of the current study support the positive role of PI3K in LTP or AMPAR trafficking indirectly; first, inhibition of PI3K in neurons prevents the formation of GRIP1/GluA2 complex, which will reduce the GluA2 surface expression (Osten et al, 2000; Zhang et al, 2011); second, depletion of PIKE-L reduces the activity of GluA2-associated PI3K, and thereby reduces the GluA2 surface retention.
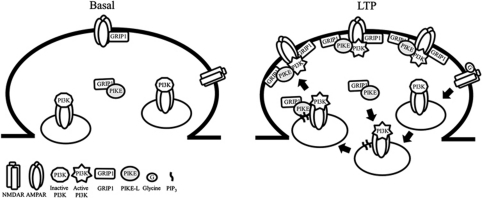
Our data also demonstrate that PIKE-L is critical for GluA2 surface expression during LTP through two mechanisms. First, PIKE-L functions as an enhancer in triggering GluA2/GRIP1 interaction. Since GRIP1 is a critical factor for AMPAR surface retention (Dong et al, 1997; Hoogenraad et al, 2005), the increased association between PIKE-L and GluA2 during glycine stimulation thus provides an extra docking site for GRIP1 to the AMPAR complex. As such, the AMPAR is remained on the cell surface to facilitate LTP induction. Second, PIKE-L is essential for the PI3KGluA2 activation. Although it has been reported that activation of synaptic NMDAR with glycine specifically stimulates the PI3KGluA2, which is essential for AMPAR membrane insertion (Man et al, 2003), the molecular mechanism of how activated-PI3K triggers such receptor trafficking remains elusive. We provide compelling evidence that association of PIKE-L with AMPAR complex substantiates PI3K activation, which is the prerequisite for subsequent GRIP1 association and its cell-surface retention. Activation of PI3KGluA2 by glycine serves as a signal to recruit PIKE-L and GRIP1 to GluA2 and the association of PIKE-L with GluA2 potentiates the PI3KGluA2 activity, thus leading to the membrane delivery of AMPAR and its surface retention (Figure 7). It is noteworthy that phospholipase Cγ (PLCγ1), the guanine exchange factor of PIKE (Ye et al, 2002), interacts with NMDAR (Gurd and Bissoon, 1997; Horne and Dell’Acqua, 2007) and is important in inducing synaptic plasticity (Reyes-Harde and Stanton, 1998; Choi et al, 2005). Though further experimental evidence is necessary, it would be logical to infer that PLCγ1-PIKE-L might represent an additional molecular linkage coupling NMDAR and PI3KGluA2 activations.
Figure 7.
Proposed functions of PIKE-L during LTP formation. PIKE-L complexes with GRIP1 under basal condition at the excitatory synapses. During NMDAR-dependent LTP (e.g. TBS or glycine stimulation), the activation of NMDAR triggers the GluA2-associated PI3K activation. The newly formed PIP3 serves as a signal for the tethering of PIKE-L/GRIP1 complex to the GluA2 of the intracellular pool, where PIKE-L sustains the activity of GluA2-associated PI3K. The PIKE-L/GRIP1/GluA2 complex will then be transported to the synaptic surface, where the GRIP1 promotes the anchorage of GluA2 on the cell surface to facilitate the formation of LTP.
AMPAR exists as a large receptor complex in the excitatory synapse. We found that PIKE-L is a new component in this receptor entirety, which represents a new functional protein in modulating the glutamate receptors signalling. We have reported previously that PIKE-L interacted with Homer and formed a complex with mGlu1, when the receptor was activated (Rong et al, 2003). PIKE-L associates with GluA2 directly but requires homer for its interaction with mGlu1 (Rong et al, 2003). It is interesting to note that 4.1 N, an interaction partner of PIKE (Ye et al, 2000), associates with AMPAR subunit GluA1 and anchors the AMPAR on the plasma membrane by coupling the receptor to the cytoskeleton (Shen et al, 2000; Lin et al, 2009). It is unknown if PIKE-L/4.1 N interaction has any physiological significance on the AMPAR function, but it is clear that PIKE-L is an important effector in the glutamate receptors complex.
We have also identified PIKE-L as a critical factor in mediating AMPA-induced cellular PI3K activity. Although it is reported that AMPA protects cultured neurons from apoptosis through the PI3K–Akt pathway (Wu et al, 2004; Nishimoto et al, 2008), the mechanism of how AMPAR activates PI3K is unknown. PI3K associates directly with the GluA2 subunit of AMPAR but this receptor-associated PI3K could only be activated by glycine but not AMPA (Man et al, 2003), suggesting that the AMPAR-tethered PI3K is not responsible for the AMPA-induced PI3K activation. In agreement with previous report, we have demonstrated that AMPA challenge to the cortical neurons increases the PI3Ktotal activity, which is PIKE-L dependent. Therefore, our results indicate that PIKE-L is not only involved in glycine-induced PI3KGluA2 activation, but also in AMPA-stimulated total PI3Ktotal activation.
In all, our data support that PIKE is critical for PI3KGluA2 activation and surface insertion of AMPAR in response to glycine treatment. Moreover, PIKE is indispensible for PI3K activation mediated by various glutamate receptors including mGlu1 and AMPAR, supporting its important roles from LTP formation to neuronal survival.
Materials and methods
Y2H screening
Y2H screen was performed with the MATCH-MAKER Two-hybrid System 2 (Clontech, USA). The GTPase domain of PIKE was subcloned downstream of the Gal4 DNA-binding domain in pAS2-1 and was used as bait to screen a human fetal brain cDNA Library in pACT2.
Co-immunoprecipitation and in vitro binding assay
HEK293 cells, cultured rat neurons or mouse brain tissues cells were lysed to perform co-immunoprecipitation and in vitro binding assays as reported (Ye et al, 1999). For PH domain interfering experiment, tissue lysates (1 mg) were incubated with the GST or GST–PIKE-L PH domain (50 μg) for 4 h at 4 °C. Control IgG or anti-PIKE-L N-terminus antibody was then added to the lysates and the immunoprecipitation was performed at 4 °C for 16 h.
Immunofluorescent staining
For cell surface GluA2 staining was performed as reported (Man et al, 2003). Images were acquired using a × 63 objective lens on Nikon C1 laser scanning confocal system. The 3D stack series of images were taken on dendritic regions of a neuron and then projected to 2D images.
In vitro PI3K assay
GluA2 or p110α were immunoprecipitated to determine total or AMPAR-associated PI3K activity as described (Ye et al, 2000).
Cell surface biotinylation assay
Cell surface GluA2 expression was detected by total cell surface biotinylation followed by NeutrAvidin pull down (Pierce, USA) as reported (Man et al, 2007).
Generation of PIKE knockout mice
Heterozygous PIKE knockout C57BL/6 mice with a targeted deletion of exons 3–6 of CENTG1 were generated under contract by Ozgene (Australia). All animal experiments were performed according to the care of experimental animal guideline and approved by the Institutional Animal Care and Use Committee from Emory University.
Immunohistochemical staining
Brain tissues were fixed in 4% paraformaldehyde, paraffin embedded and sectioned (8 μm) in standard procedures. After serial rehydration and permeabilization in 0.1% TBST, sections were immunostained using specific antibodies as indicated and counterstained with haematoxylin using Zymed Histostain SP kit (Invitrogen, USA).
Recording of hippocampal slices
Hippocampal slices were prepared as described previously (Chen et al, 2010). fEPSPs were evoked in the CA1 stratum radiatum by stimulating schaffer collaterals with a two-concentric bipolar stimulating electrode (FHC, ME) and recorded in current-clamp by the Axon MultiClamp 700B amplifier with ACSF-filled glass pipettes. LTP was induced using TBS or HFS as described previously (Larson et al, 1986; Opazo et al, 2003). For whole-cell recording, CA1 neurons were visualized with infrared optics using an upright microscope equipped with a × 40 water-immersion lens (Olympus, BX51WI) and infrared-sensitive CCD camera.
Glycine-induced LTP in hippocampal neurons
E18 or P0 hippocampal neurons were cultured in vitro for 12–17 days. Neurons were recorded in whole-cell configuration at room temperature. In some experiments, GST or GST–PH (final concentration at 5 μg/ml) was included in intracellular recording solution prior to glycine application.
Statistical analysis
Results were expressed as mean±s.e.m. and were considered significant when P<0.05. Statistical analysis of the data was performed using either Student's t-test or two-way ANOVA followed by Bonferroni post tests by the computer program GraphPad Prism (GraphPad Software, USA).
Detailed experimental procedures and reagents information are in the supplemental data available online.
Supplementary Material
Acknowledgments
We thank Dr Jun Xia at Hong Kong University of Science and Technology (Hong Kong, China) for the myc–GRIP1, pRK5-GluA1 and pRK5-GluA2 plasmids and Dr Edward B Ziff at New York University School of Medicine (NY, USA) for the myc–GluA2 WT and myc–GluA2 AVKI plasmids. This work was supported by Grant NIH R01045627 to KYe.
Author contributions: CBC and YC designed and performed the experiments, analysed the data and wrote the manuscript; XL, XT and CWL performed the experiments, LM analysed the data and wrote the manuscript; KY designed the experiments, analysed the data and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahn JY, Rong R, Kroll TG, Van Meir EG, Snyder SH, Ye K (2004) PIKE (phosphatidylinositol 3-kinase enhancer)-A GTPase stimulates Akt activity and mediates cellular invasion. J Biol Chem 279: 16441–16451 [DOI] [PubMed] [Google Scholar]
- Arendt KL, Royo M, Fernandez-Monreal M, Knafo S, Petrok CN, Martens JR, Esteban JA (2010) PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane. Nat Neurosci 13: 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39 [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B (1992) Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8: 189–198 [DOI] [PubMed] [Google Scholar]
- Capocchi G, Zampolini M, Larson J (1992) Theta burst stimulation is optimal for induction of LTP at both apical and basal dendritic synapses on hippocampal CA1 neurons. Brain Res 591: 332–336 [DOI] [PubMed] [Google Scholar]
- Chan CB, Ye K (2007) PIKE GTPase are phosphoinositide-3-kinase enhancers, suppressing programmed cell death. J Cell Mol Med 11: 39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, Lu YS, Zhu XH, Li SJ, Wu CY, Wang XM, Lai C, Xiong WC, Mei L, Gao TM (2010) ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci USA 107: 21818–21823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Chang J, Jiang B, Seol GH, Min SS, Han JS, Shin HS, Gallagher M, Kirkwood A (2005) Multiple receptors coupled to phospholipase C gate long-term depression in visual cortex. J Neurosci 25: 11433–11443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N (2009) Translational control of long-lasting synaptic plasticity and memory. Neuron 61: 10–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL (1997) GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386: 279–284 [DOI] [PubMed] [Google Scholar]
- Genoux D, Montgomery JM (2007) Glutamate receptor plasticity at excitatory synapses in the brain. Clin Exp Pharmacol Physiol 34: 1058–1063 [DOI] [PubMed] [Google Scholar]
- Gurd JW, Bissoon N (1997) The N-methyl-D-aspartate receptor subunits NR2A and NR2B bind to the SH2 domains of phospholipase C-gamma. J Neurochem 69: 623–630 [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M, Sheng M (2005) GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat Neurosci 8: 906–915 [DOI] [PubMed] [Google Scholar]
- Horne EA, Dell’Acqua ML (2007) Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression. J Neurosci 27: 3523–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S (2006) Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci 23: 3375–3384 [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 529–531 [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G (1986) Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res 368: 347–350 [DOI] [PubMed] [Google Scholar]
- Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL (2009) Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci 12: 879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M (1998) Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci USA 95: 7097–7102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Jang SW, Liu X, Cheng D, Peng J, Yepes M, Li XJ, Matthews S, Watts C, Asano M, Hara-Nishimura I, Luo HR, Ye K (2008) Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol Cell 29: 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT (2001) Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29: 243–254 [DOI] [PubMed] [Google Scholar]
- Lu W, Ziff EB (2005) PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron 47: 407–421 [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL (2007) Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropioni. Proc Natl Acad Sci USA 104: 3579–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D’Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, MacDonald JF, Wang YT (2003) Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron 38: 611–624 [DOI] [PubMed] [Google Scholar]
- Matsuda S, Mikawa S, Hirai H (1999) Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem 73: 1765–1768 [DOI] [PubMed] [Google Scholar]
- Muller D, Toni N, Buchs PA (2000) Spine changes associated with long-term potentiation. Hippocampus 10: 596–604 [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Kihara T, Akaike A, Niidome T, Sugimoto H (2008) alpha-Amino-3-hydroxy-5-methyl-4-isoxazole propionate attenuates glutamate-induced caspase-3 cleavage via regulation of glycogen synthase kinase 3beta. J Neurosci Res 86: 1096–1105 [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM (1998) NSF binding to GluR2 regulates synaptic transmission. Neuron 21: 87–97 [DOI] [PubMed] [Google Scholar]
- Opazo P, Watabe AM, Grant SG, O’Dell TJ (2003) Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. J Neurosci 23: 3679–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB (2000) Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron 27: 313–325 [DOI] [PubMed] [Google Scholar]
- Palmer CL, Cotton L, Henley JM (2005) The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev 57: 253–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL (2007) LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53: 703–717 [DOI] [PubMed] [Google Scholar]
- Perestenko PV, Henley JM (2003) Characterization of the intracellular transport of GluR1 and GluR2 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem 278: 43525–43532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Harde M, Stanton PK (1998) Postsynaptic phospholipase C activity is required for the induction of homosynaptic long-term depression in rat hippocampus. Neurosci Lett 252: 155–158 [DOI] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K (2003) PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci 6: 1153–1161 [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF (1998) The tetrameric structure of a glutamate receptor channel. Science 280: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W (2002) Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci 22: 3359–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Vissel B, Petralia RS, Wang YX, Chang K, Royle GA, Wang CY, O’Gorman S, Heinemann SF, Wenthold RJ (2003) Aberrant formation of glutamate receptor complexes in hippocampal neurons of mice lacking the GluR2 AMPA receptor subunit. J Neurosci 23: 9367–9373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SD, Carvalho AL, Caldeira MV, Duarte CB (2009) Regulation of AMPA receptors and synaptic plasticity. Neuroscience 158: 105–125 [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N (2002) Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature 417: 83–87 [DOI] [PubMed] [Google Scholar]
- Shen L, Liang F, Walensky LD, Huganir RL (2000) Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association. J Neurosci 20: 7932–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284: 1811–1816 [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL (2002) Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25: 578–588 [DOI] [PubMed] [Google Scholar]
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, Weinberg RJ, Ziff EB (1998) Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron 21: 581–591 [DOI] [PubMed] [Google Scholar]
- Tang X, Jang SW, Okada M, Chan CB, Feng Y, Liu Y, Luo SW, Hong Y, Rama N, Xiong WC, Mehlen P, Ye K (2008) Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat Cell Biol 10: 698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhu D, Jiang X, Okagaki P, Mearow K, Zhu G, McCall S, Banaudha K, Lipsky RH, Marini AM (2004) AMPA protects cultured neurons against glutamate excitotoxicity through a phosphatidylinositol 3-kinase-dependent activation in extracellular signal-regulated kinase to upregulate BDNF gene expression. J Neurochem 90: 807–818 [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Valtschanoff JG, Naisbitt S, Dunah AW, Kim E, Standaert DG, Weinberg R, Sheng M (1999) Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J Neurosci 19: 6528–6537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL (1999) Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 22: 179–187 [DOI] [PubMed] [Google Scholar]
- Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, Hurt KJ, Bae SS, Suh PG, Snyder SH (2002) Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415: 541–544 [DOI] [PubMed] [Google Scholar]
- Ye K, Compton DA, Lai MM, Walensky LD, Snyder SH (1999) Protein 4.1N binding to nuclear mitotic apparatus protein in PC12 cells mediates the antiproliferative actions of nerve growth factor. J Neurosci 19: 10747–10756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, Blackshaw S, Ferris CD, Snyder SH (2000) Pike. A nuclear GTPase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell 103: 919–930 [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang Y, Chi Z, Keuss MJ, Pai YM, Kang HC, Shin JH, Bugayenko A, Wang H, Xiong Y, Pletnikov MV, Mattson MP, Dawson TM, Dawson VL (2011) The AAA(+) ATPase thorase regulates AMPA receptor-dependent synaptic plasticity and behavior. Cell 145: 284–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Fan JS, Zhang M (2001) Interdomain chaperoning between PSD-95, Dlg, and Zo-1 (PDZ) domains of glutamate receptor-interacting proteins. J Biol Chem 276: 43216–43220 [DOI] [PubMed] [Google Scholar]
- Ziff EB (2007) TARPs and the AMPA receptor trafficking paradox. Neuron 53: 627–633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.