Figure 5.
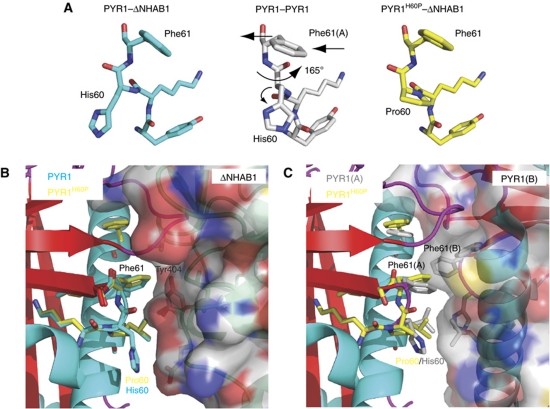
Structure of the PYR1H60P mutant and its effect on the dimerization interface. The structure of PYR1H60P in complex with ΔNHAB1 and (+)ABA was obtained by X-ray crystallography (3ZVU). (A) Detail of the conformation of peptide backbone at position 60 and its effect on Phe61 in the wt PYR1–ΔNHAB1 complex (3QN1) (left, blue), the PYR1 dimer (3K90) (centre, grey) and the PYR1H60P–ΔNHAB1 (right, yellow). Arrows indicate the rearrangement of the peptide backbone around His60 and the displacement of Phe61 in dimeric PYR1. (B) Detail of the receptor–PP2C interface in the wt PYR1–ΔNHAB1 and the PYR1H60P–ΔNHAB1 complexes. Wt PYR1 is shown as in Figure 1 with relevant residues at the α4/β2 loop in blue. The corresponding residues of PYR1H60P are shown in yellow. The backbone of ΔNHAB1 is shown in green with semitransparent molecular surface. As can be appreciated except for the His/Pro substitution, both PYR1 proteins form virtually identical interfaces with Phe61 occupying a pocket at the centre of the receptor–phosphatase interface. (C) The structure of PYR1H60P (yellow) from the model above was overlaid on subunit A of the PYR1 dimer (backbone as in Figure 1 and side chains in grey). The structure of the second subunit in the PYR1 dimer is shown with semitransparent molecular surface and critical side chains in grey. The modifications in the conformation of the α4/β2 loop and the outward displacement of Phe61 in dimeric PYR1 leave space to accommodate the side chain of Phe61(B) of the second subunit in the dimer, which occupies the centre of the dimerization interface. The position of Phe61 from PYR1H60P (yellow) partially overlaps with that of Phe61(B). The modification of the backbone torsion angles at position 60 in the PYR1 dimer (A) produces an outward displacement of Phe61 preventing a steric clash between the two Phe61 residues at the dimer interface. The introduction of a proline at position 60 limits backbone flexibility hindering this rearrangement.