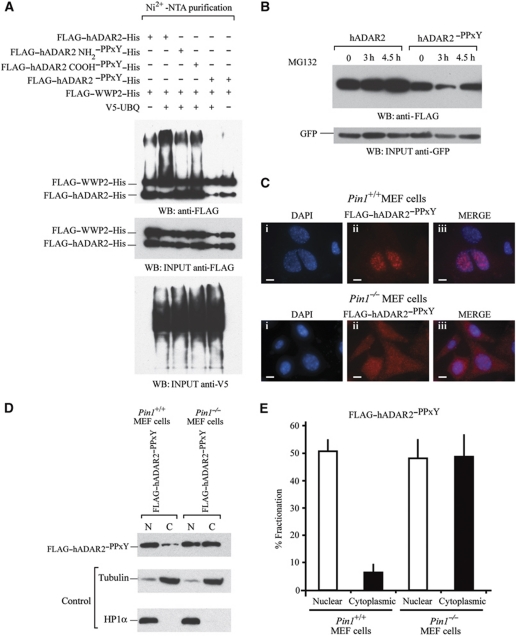
Figure 7.
WWP2 is required for ADAR2 ubiquitination and subsequent degradation in the cytoplasm. (A) In vivo ubiquitination assays. FLAG–ADAR2–His, FLAG–WWP2–His and V5-UBQ were transfected in HEK293T cells followed by purification of ubiquitination complexes from lysates with Ni2+-NTA. In lane 1, cotransfection was with FLAG–ADAR2–His, FLAG–WWP2–His. Lane 2, cotransfection was with FLAG–ADAR2–His, FLAG–WWP2–His and V5-UBQ. Lane 3, cotransfection of FLAG–ADAR2 NH2–PPxY–His, FLAG–WWP2–His and V5-UBQ. Lane 4, cotransfection of FLAG–ADAR2 COOH–PPxY–His, FLAG–WWP2–His and V5-UBQ. Lane 5, cotransfection of FLAG–ADAR2–PPxY–His (double mutant), FLAG–WWP2–His and V5-UBQ. Lane 6 is the same as lane 5 without the addition of V5-UBQ and is the negative control. (Middle panel) Immunoblot of input proteins detected with anti-FLAG antibody. (Lower panel) Immunoblot of V5-UBQ present in the purified complex detected with anti-V5 antibody. (B) Immunoblot at 24 h following transfection of FLAG–ADAR2 and FLAG–ADAR2–PPxY with 20 μM MG132 to inhibit protein degradation. The proteasomal inhibitor MG132 was added to both and a time course from 0 to 4.5 h was performed in HeLa cells. Cell lysates were normalized to GFP levels (lower panel). (C) Immunofluorescence of Pin1+/+and Pin1−/− MEF cells cotransfected with GluR2 and FLAG–ADAR2–PPxY (double mutant). (i) DAPI staining of nuclei. (ii) Anti-FLAG–ADAR2–PPxY (red). (iii) Merge of DAP1 and FLAG. Scale bar, 10 μm. All photographs were taken at the same exposure. (D) Nuclear and cytoplasmic fractionation of FLAG–ADAR2–PPxY in Pin1+/+and Pin1−/− MEF cells. (Lower panels) Immunoblot of fractionated MEF cells with tubulin as a cytoplasmic marker and HP1α as a nuclear marker. (E) Quantification of (D).