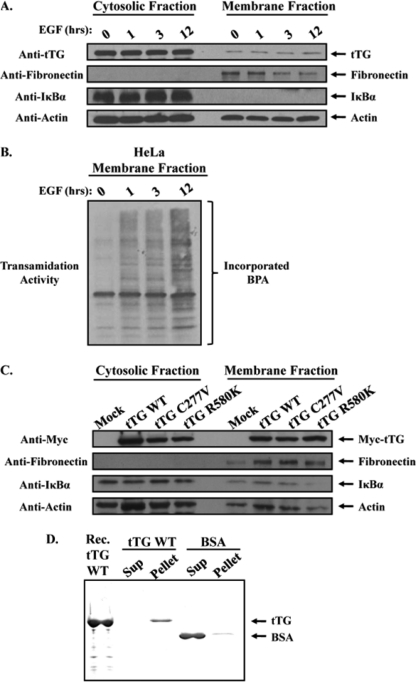
FIGURE 2.
A pool of tTG is constitutively associated with the plasma membrane. Serum-starved HeLa cells that had been treated without (lane 0) or with EGF for increasing lengths of time, as indicated, were homogenized and then subjected to differential centrifugation to separate cytosolic and membrane fractions. A, the cellular fractions were immunoblotted with tTG, fibronectin, IκBα, and actin antibodies. B, the same membrane fractions were also assayed for tTG transamidation activity by determining the incorporation of BPA into lysate proteins. C, actively growing HeLa cells that had been either mock transfected without DNA (Mock) or transfected with various Myc-tagged forms of tTG including wild-type (tTG WT), a transamidation-defective mutant (tTG C277V), or a GTP-binding-defective mutant (tTG R580K) were homogenized and then subjected to differential centrifugation to isolate cytosolic and membrane fractions. The fractions were immunoblotted with Myc, fibronectin, IκBα, and actin antibodies. D, synthetic liposomes were prepared by extrusion, and then equal amounts of this preparation were combined with either 5 μg of recombinant tTG (tTG WT) or 5 μg of BSA. After a 15-min incubation, the liposomes were pelleted by centrifugation, and the resulting supernatant (Sup) and liposome (Pellet) fractions were resolved by SDS-PAGE. The gel was then stained with Quick Blue to detect the proteins. A lane containing recombinant tTG (Rec. tTG WT) was included as a standard.