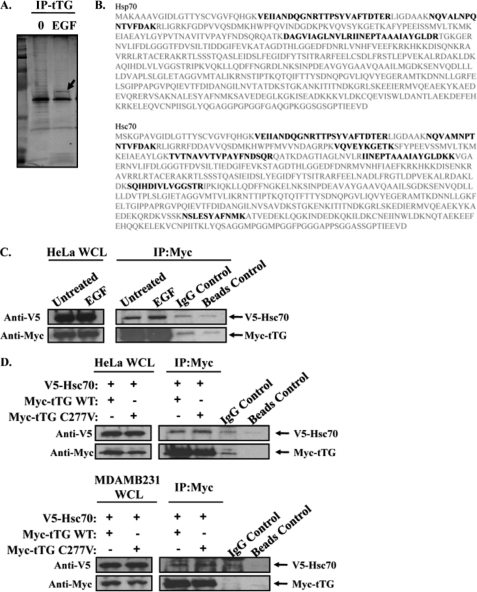
FIGURE 3.
Hsp70 interacts with plasma membrane-associated tTG. A, serum-starved HeLa cells that had been treated without (lane 0) or with EGF for 12 h were homogenized and then subjected to differential centrifugation to isolate the membrane components of the cells. Immunoprecipitations with a tTG antibody (IP:tTG) were performed on the membrane extracts, and the resulting immunocomplexes were resolved by SDS-PAGE. The gel was then stained with Colloidal Blue to detect the proteins that co-immunoprecipitated with tTG. One protein band (Mr = ∼70 kDa), denoted with an arrow, was determined to contain two isoforms of the heat shock protein 70 family, Hsp70 and Hsc70, by mass spectrometry. B, the protein sequences of human Hsp70 and Hsc70 are shown in gray. The peptide fragments identified by mass spectrometry are shaded in black. C, immunoprecipitations with a Myc antibody were performed on the extracts of HeLa cells ectopically expressing V5-tagged Hsc70 and Myc-tagged tTG, treated without (Untreated) or with EGF. The whole cells lysates (WCL) and the resulting immunocomplexes (IP:Myc) were immunoblotted with V5 and Myc antibodies. Nonspecific mouse IgG control antibody and beads-only control immunoprecipitations were also performed on the extracts to show that the Hsc70-tTG interaction was specific. D, immunoprecipitations with a Myc antibody were performed on the extracts of actively growing HeLa (top panels) and MDAMB231 cells (bottom panels) that were ectopically expressing V5-tagged Hsc70 and a Myc-tagged form of either wild-type tTG (tTG WT) or a transamidation-defective form of tTG (tTG C277V). The whole cell lysates (WCL) and the resulting immunocomplexes (IP:Myc) were immunoblotted with V5 and Myc antibodies. Nonspecific mouse IgG control antibody and beads-only control immunoprecipitations were performed on the extracts collected from HeLa and MDAMB231 cells expressing V5-Hsc70 and Myc-tTG WT to show that the Hsc70-tTG interaction was specific.