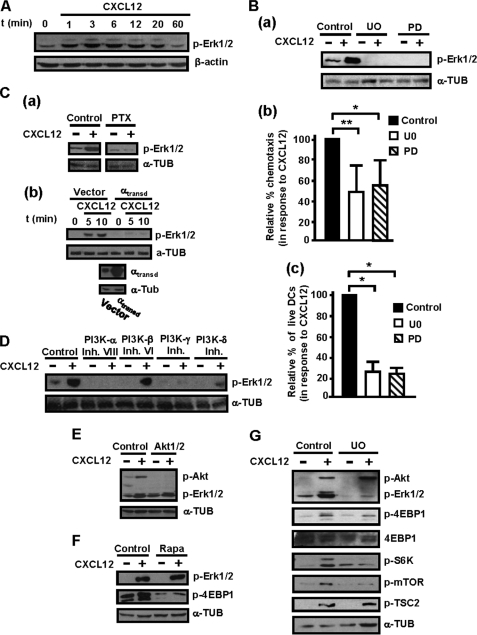
FIGURE 5.
ERK1/2 regulate CXCL12-mediated chemotaxis and survival downstream of Gi, Gβγ, PI3K-α, -γ, -δ isoforms, but not of PI3Kβ, Akt, and mTORC1 in human maDCs. A, DCs were stimulated for the indicated times with CXCL12 as in Fig. 3A, and aliquots were taken to analyze the level of phosphorylated/active ERK1/2 (p-ERK1/2) by Western blotting. β-Actin levels show equal loading of the gels. A representative experiment of three performed is shown. A and Fig. 3A correspond to the same experiment, and thus similar β-actin control is used. Ba, DCs suspended in 0.1% BSA in RPMI 1640 medium were left untreated (Control) or pretreated with the ERK1/2 inhibitors UO126 (UO) or PD0325901 (PD), both at 5 μm, for 1 h. Subsequently, DCs were stimulated or not with CXCL12. After 3 min of stimulation with CXCL12, aliquots were taken to analyze the level of phosphorylated/active ERK1/2 or α-tubulin (α-TUB). A representative experiment of four performed is shown. b, DCs untreated or pretreated with UO126 or with PD0325901, as in a, were allowed to migrate toward CXCL12. Migration was analyzed in Transwell setting as in Fig. 4Aa. Results represent the mean ± S.E. (error bars; n = 6). **, p < 0.001. c, DCs were suspended in 0.1% BSA in RPMI 1640 medium plus CXCL12 and then kept untreated or treated with UO126 or PD0325901, both at 5 μm, for 40 h. Apoptotic DCs were determined as in Fig. 4Ab. Results represent the mean ± S.E. (n = 8). **, p < 0.001. Ca, DCs were left untreated or pretreated with PTX and, subsequently, were stimulated or not with CXCL12 for 3 min (as in Fig. 3B). Aliquots were taken to analyze the level of phosphorylated/active ERK1/2 and α-tubulin. A representative experiment of four performed is shown. b upper, DCs were transfected either with vector or with α-transducin (αtransd). 18 h after transfection, a similar number of live vector- and α-transducin-transfected DCs were taken and then stimulated for the indicated times with CXCL12. The levels of phosphorylated/active ERK1/2 were analyzed by Western blotting. Lower, expression of α-transducin was analyzed by Western blotting. α-Tubulin levels show equal loading of the gels. A representative experiment of four performed is shown. D, DCs were suspended in 0.1% BSA/RPMI 1640 medium and then kept untreated or pretreated with PI3K-α, PI3K-β, PI3K-γ, or PI3K-δ inhibitors for 1 h as in Fig. 3C. Subsequently, DCs were stimulated or not with CXCL12 for 3 min. Subsequently, aliquots were taken to analyze the level of phosphorylated/active ERK1/2 (p-ERK1/2). α-Tubulin levels show equal loading of the gels. D and 3C correspond to the same experiment, and thus a similar β-actin control is used. A representative experiment of six performed is shown. E, DCs were suspended in 0.1% BSA/RPMI 1640 medium and then kept untreated or pretreated with Akt inhibitor for 1 h as in Fig. 3G. Subsequently, DCs were stimulated or not with CXCL12. After 3 min of stimulation with the chemokine, aliquots were taken to analyze the level of phosphorylated/active Akt (p-Akt), phosphorylated/active ERK1/2 and α-tubulin. A representative experiment of four performed is shown. F, DCs were left untreated or pretreated with a specific mTORC1 inhibitor (rapamycin, 100 nm) for 1 h. Subsequently, the DCs were stimulated or not with CXCL12. After 3 min of stimulation with the chemokine, aliquots were taken to analyze the level of phosphorylated/active ERK1/2, phosphorylated 4E-BP1, and α-tubulin. A representative experiment of four performed is shown. G, DCs were left untreated or pretreated with a specific ERK1/2 inhibitor (UO126, 5 μm) for 60 min. Subsequently, DCs were stimulated or not with CXCL12. After 3 min of stimulation with CXCL12, aliquots were taken to analyze the level of phosphorylated TSC2, phosphorylated/active Akt, phosphorylated/active ERK1/2, phosphorylated 4E-BP1, phosphorylated S6K, phosphorylated mTOR. α-Tubulin and 4E-BP1 levels show equal loading of the gels. A representative experiment of eight performed is shown.