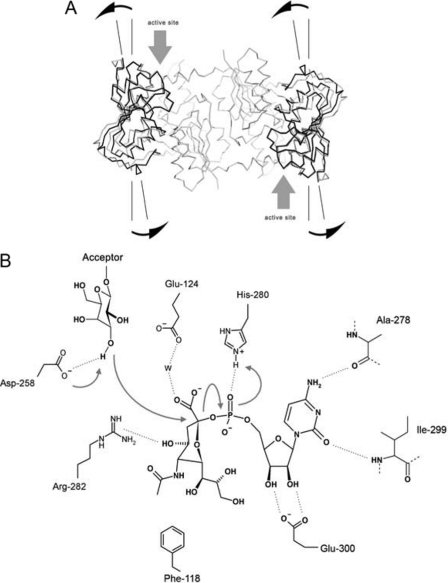
FIGURE 3.
A, conformational change upon CMP-3F(axial)-Neu5Ac binding is shown. Comparison of the apoNST and the donor analog-bound structures reveals an approximate 10° rotation on a linear axis formed by the Cαs of Leu-253, Val-277, Tyr-318, and Tyr-337 localized to the central β-sheet in the C-terminal domain using the model-building program Coot. This conformational change effectively pries open the N- and C- interdomain cleft (highlighted with gray arrows), making the active site more accessible to substrates. B, shown is the proposed catalytic mechanism for NST. The transfer reaction occurs between the terminal galactosyl residue of the acceptor substrate and the anchored sialic acid moiety that is covalently linked to the rigidly coordinated CMP group in NST. This CMP binding mode is strengthened by bifurcated hydrogen bonds between Glu-300 and the ribosyl hydroxyl groups and by the specific recognition of the cytidine base that is established through hydrogen bonds with the main chain amide and carbonyl groups of Ile-299 and Ala-278, respectively. The unique geometry of the sialic acid moiety in the CMP-3F(axial)-Neu5Ac·NST complex is stabilized through several interactions including a hydrogen bond from the His-280 side chain to the CMP phosphate of the donor, a water-mediated hydrogen bond bridging the sialyl carboxylate to the side chain of Glu-124, and a hydrogen bond between the C4 hydroxyl group of sialic acid and the guanidinyl group of Arg-282. These interactions fix the apparent half-chair sialyl ring geometry in NST and orient the anomeric center toward the entrance of the active site cavity, ready for transfer to the acceptor substrate. Activation of the acceptor nucleophile (deprotonation of the hydroxyl group on the galactosyl acceptor) is carried out by the proposed general base Asp-258, which is located at the rim of the active site entrance and next to the C2 anomeric center on sialic acid. A concerted mechanism is presented in the figure and follows the classical SN2 type nucleophilic substitution reaction, which involves the development of sp2-like hybridization at the sialyl C2 position, and formation of a planar oxocarbenium ion in the transition state. Inversion of stereochemistry at the C2 reaction center that leads to formation of an α-configured glycosidic bond in the sialylated galactosyl product is enhanced by general acid assistance from the proximal His-280, which stabilizes the developing negative charge on the phosphate leaving group.