FIGURE 6.
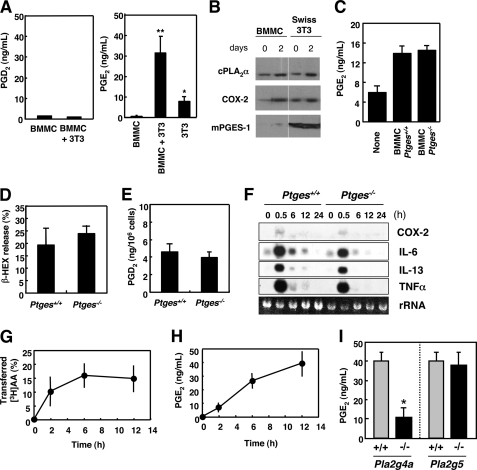
cPLA2α in mast cell is coupled with mPGES-1 in fibroblasts to provide anti-allergic PGE2. A, BMMCs alone, BMMCs plus Swiss 3T3 cells, and Swiss 3T3 alone were cultured for 2 days, and the resultant supernatants were taken for enzyme immunoassay for PGD2 (left panel) or PGE2 (right panel). Means ± S.D., n = 6; *, p < 0.01 versus BMMCs, or Swiss 3T3 cells alone and **, p < 0.01 versus BMMCs alone. B, expression of PGE2 biosynthetic enzymes in BMMCs and Swiss 3T3 cells before and 2 days after coculture, as assessed by immunoblotting. C, PGE2 production by Swiss 3T3 cells alone and those cocultured with Ptges+/+ or Ptges−/− BMMCs (mean ± S.D., n = 2). D and E, BMMCs from Ptges+/+ and Ptges−/− mice were sensitized with IgE and stimulated with Ag for 15 min to assess β-HEX exocytosis (D) and PGD2 generation (E) (mean ± S.D., n = 8-∼12). F, total RNAs extracted from Ptges+/+ and Ptges−/− BMMCs after stimulation with Ag for the indicated periods were subjected to Northern blotting for COX-2 and various cytokines (IL-6, IL-13, and TNFα). rRNA, 28 S ribosomal RNA visualized in an agarose gel with ethidium bromide. A representative result of three independent experiments is shown. G, [3H]AA-prelabeled BMMCs were cocultured with Swiss 3T3 cells, and percentage transfer of [3H]AA to Swiss 3T3 cells relative to total incorporation of [3H]AA into BMMCs was monitored over time (mean ± S.D., n = 4). H, time course of PGE2 synthesis by BMMC-Swiss 3T3 coculture (mean ± S.D., n = 4). I, BMMCs obtained from Pla2g4a−/− (n = 8) and Pla2g5−/− (n = 4) mice and their littermate wild-type controls were cocultured with Swiss 3T3 cells for 12 h to assess the coculture-dependent increase in PGE2 release (mean ± S.D., *, p < 0.05 versus Pla2g4a+/+ control).