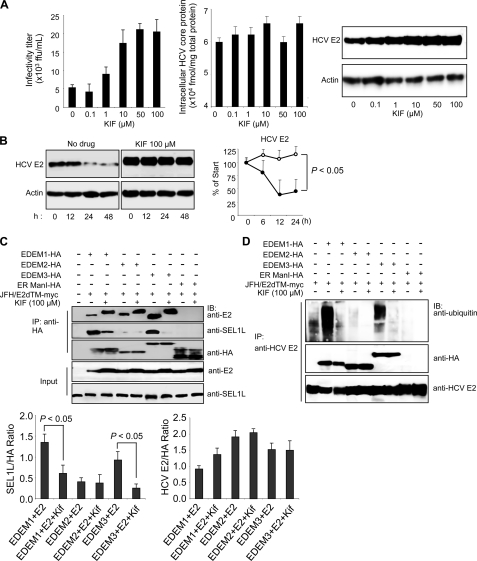
FIGURE 5.
Effect of KIF on HCV production and stability of E2. A, extracellular HCV titer, intracellular HCV core protein expression, and steady-state level of HCV E2 in HuH-7 cells treated with different concentrations of KIF. B, CHX-based HCV protein stability assay of HCV E2 protein in KIF-treated cells as described in Fig. 3E. E2 protein levels normalized to actin levels are shown in the graph on the right. The open and filled circles indicate KIF-treated and nontreated cells, respectively. The mean ± S.D. (error bars) of two independent experiments are shown. C, binding of EDEMs and ER ManI with HCV E2 and SEL1L in 293T cells in the absence or presence of KIF. 293T cells were seeded in 6-well plates at a density of 3 × 105 cells/well. After overnight incubation, the cells were co-transfected with plasmids carrying HCV E2-myc (1 μg) and EDEM1-HA, EDEM2-HA, EDEM3-HA, or ER ManI-HA proteins (1 μg each). After 6 h, the culture medium was replaced with fresh or KIF-containing medium (100 μm). Forty-eight hours later, the cells were harvested and immunoprecipitated (IP) with anti-HA antibodies, after which Western blotting (IB) was performed with the indicated antibodies. Specific signals were quantified by densitometry, and the ratio between HCV E2 and HA (right graph) and between SEL1L and HA (left graph) in the same lanes is plotted on the graphs. The mean ± S.D. of three independent experiments are shown. D, EDEM protein-mediated ubiquitylation of HCV E2 protein in 293T cells in the absence or presence of KIF. The experimental procedure was the same as that described in Fig. 5C, except that immunoprecipitation was performed with anti-HCV E2 antibodies.