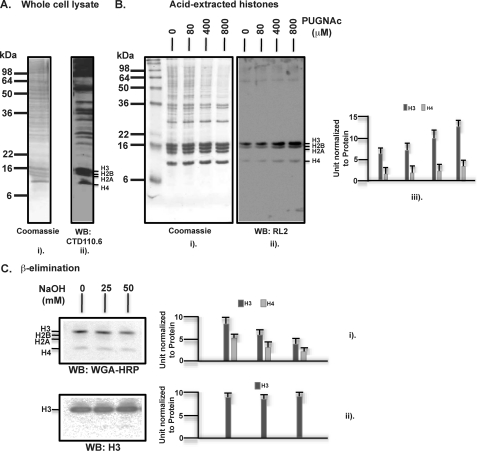
FIGURE 1.
Human histones are post-translationally modified by glycosylation, specifically O-GlcNAcylation. A, whole cell lysates were prepared from cultured HEK293 cells that were immediately denatured in SDS-PAGE sampling buffer at 95 °C for 5 min. After electrophoresis through a 15% SDS-polyacrylamide gel, Western blotting (WB) was performed with the mouse monoclonal antibody CTD110.6, specific to O-GlcNAc. B, histones were prepared by acid extraction from HEK293 cells as described under “Experimental Procedures.” O-(2-Acetamido-2-deoxy-d-glucopyranosylidene) amino N-phenylcarbamate (PUGNAc) was included at the indicated concentrations in all the buffers to inhibit O-GlcNAcase that removes O-GlcNAc. After SDS-PAGE of extracted histones, Western blotting was performed with the mouse monoclonal antibody RL2, also specific to O-GlcNAc, which is quantified three times via densitometry and presented in units normalized to the protein levels, the errors indicating means ± S.D. C, histone O-GlcNAcylation was examined for its sensitivity to mild base treatment leading to β-elimination of the O-linked GlcNAc. For this purpose, the acid-extracted histones were incubated without or with 25 and 50 mm NaOH for 30 min at 37 °C, followed by SDS-PAGE and Western blotting with a lectin probe WGA-HRP that is also specific to O-GlcNAc. A rabbit polyclonal antibody against total histone H3 was used for presenting an input control. Quantifications are presented as above.