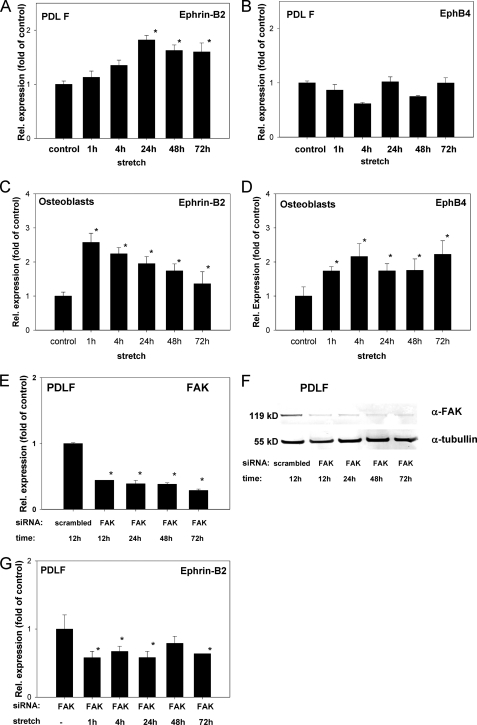
FIGURE 4.
Ephrin-B2 expression increases in PDL fibroblasts and decreases in osteoblasts exposed to mechanical strain. qRT-PCR analyses for ephrin-B2 (A) and EphB4 (B) expression after the application of mechanical strain in PDLF. Mechanical strain was applied for 1–72 h at 2.5% elongation. Significant overexpression for ephrin-B2, as compared with static control cells, was demonstrated after 24 h and sustained until 72 h (A). EphB4 was not significantly altered during the course of the experiment. Ephrin-B2 and EphB4 interactions might be evident within the osteoblast lineage. Therefore, osteoblasts were subjected to mechanical strain and tested for the transcription of ephrin-B2 and EphB4 by means of qRT-PCR (C and D). Ephrin-B2 and EphB4 expression was increased. It is noteworthy that temporal expression pattern of ephrin-B2 in osteoblasts was reciprocal to the temporal ephrin-B2 expression pattern observed in PDLF. Values are represented as mean ± S.D. *, p < 0.05 versus control, n = 3. qRT-PCR experiments were performed in triplicate. FAK might be a prominent mediator of mechano-induced alterations of ephrin-B2 expression in PDLF. siRNA against FAK was used to knock down FAK expression in PDLF. siRNA against FAK and a scrambled control siRNA were transfected to PDLF, and FAK expression was monitored on the mRNA level using qRT-PCR. E, siRNA against FAK attenuated FAK expression in PDLF after 12 h and attenuation remained significant up to 72 h. FAK attenuation mediated by siRNA was also monitored on the protein level using Western blotting against FAK (F), and probing for tubulin served as a loading control. Western blotting confirmed the siRNA-mediated knockdown of FAK in PDLF. PDLF attenuated for FAK using siRNA were subjected to mechanical strain for 1–72 h (G), and ephrin-B2 expression was assessed at discrete time points using qRT-PCR. Unlike WT PDLF, siRNA-transfected PDLF did not show an up-regulation of ephrin-B2 upon the application of mechanical strain.