FIGURE 5.
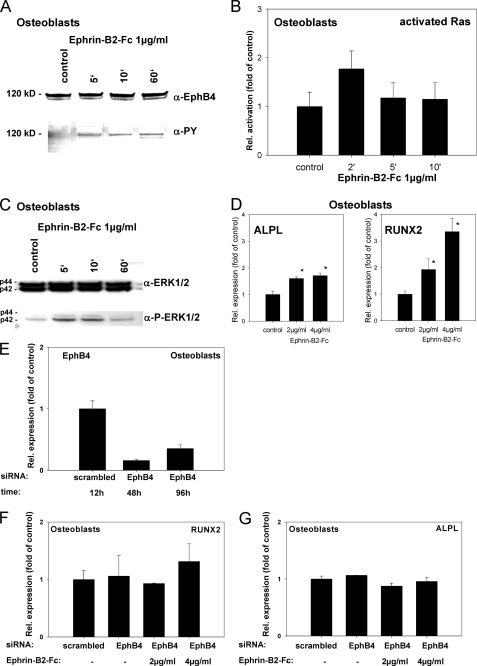
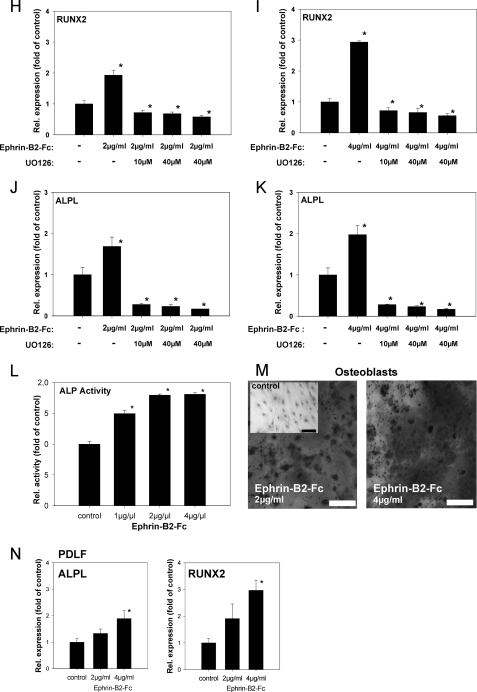
Ephrin-B2 activates Runx2 and ALPL transcription in osteoblasts of the alveolar bone via a Ras- and ERK1/2-dependent pathway. To prove that ephrin-B2-Fc causes EphB4 receptor phosphorylation in osteoblasts of the alveolar bone, osteoblasts were stimulated with 1 μg/ml ephrin-B2-Fc for 5–60 min. Tyrosine phosphorylation of the EphB4 receptor was detected after 5 min and sustained through the course of the experiment (A). To elucidate a putative signaling pathway linking ephrin-B2-Fc stimulation and downstream events, osteoblasts of the alveolar bone were stimulated with 1 μg/ml ephrin-B2-Fc for 2–10 min, and Ras activation was monitored. Ras was transiently activated after ephrin-B2-Fc stimulation (2 min) (B). Ras might transmit downstream signals via ERK1/2. To reveal a possible ERK1/2 dependence of ephrin-B2 downstream signaling, protein lysates of osteoblasts of the alveolar bone stimulated with ephrin-B2-Fc (1 μg/ml) for 5–60 min were probed with antibodies against ERK1/2 and pERK1/2. An increase in phosphorylated ERK1/2 was detected 5 min after stimulation, gradually decreasing to nearly base-line phosphorylation after 60 min (C). To detect further downstream effects of ephrin-B2-dependent signaling in osteoblasts of the alveolar bone, osteoblasts were stimulated with 2 and 4 μg/ml, respectively, of ephrin-B2-Fc for 6 days. qRT-PCR revealed the significant induction of Runx2, as well as ALPL in a dose-dependent manner (D). Although EphB4 is the only EphB receptor exhibiting a binding specificity for a single ligand, ephrin-B2 has binding affinity for other receptors of the EphB-class. To rule out the involvement of other EphB receptors for the ephrin-B2-dependent activation of Runx2 and ALP mRNA expression, we have used siRNA to knock down EphB4 in osteoblasts. siRNA against EphB4 effectively suppressed EphB4 expression in osteoblasts for up to 96 h (E). Stimulation of osteoblasts with 2 and 4 μg/ml ephrin-B2 in the presence of siRNA against EphB4 did not result in an up-regulation of Runx2 (F) or ALP (G) expression as observed in WT osteoblasts (D). The activation of ERK1/2 in osteoblasts upon ephrin-B2 stimulation (C) suggested the involvement of an ERK1/2-dependent pathway in the induction of osteoblastogenic gene expression after ephrin-B2 stimulation. We have used a specific MEK1/2 inhibitor, UO126, to block ERK1/2 activation in osteoblasts. Osteoblasts were stimulated with 2 or 4 μg/ml ephrin-B2 in the absence or presence of different concentrations of UO126. Runx2 (H and I) and ALP (J and K) were monitored by means of qRT-PCR. Already at a concentration of 10 μm, UO126 attenuated the ephrin-B2-dependent activation of Runx2 and its downstream target ALP in osteoblasts. These results suggested an involvement of an ERK1/2-dependent pathway in the ephrin-B2-dependent regulation of Runx2 in osteoblasts. Increased ALP transcription was accompanied by an increase in ALP activity (L). Alizarin Red staining for calcified nodules in osteoblasts stimulated for 6 days with ephrin-B2-Fc (2 and 4 μg/ml) further confirmed the osteoblastogenic effects of ephrin-B2-Fc stimulation. An untreated control is shown as an inset. Scale bar, 20 μm (M). Subpopulations of PDLF might contribute to osteogenesis at tension sites in orthodontic tooth movement. Therefore, we have tested for the expression of Runx2 and ALP in PDLF after stimulation with ephrin-B2-Fc by means of qRT-PCR. Both ALP and Runx2 transcription were up-regulated in a dose-dependent manner in PDLF (N). Up-regulation of ALPL and Runx2 transcription reached significance after stimulation with 4 μg/ml of ephrin-B2-Fc. Values are represented as mean ± S.D. *, p < 0.05 versus control, n = 3.