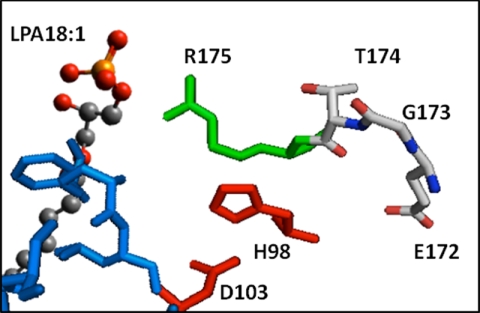
FIGURE 11.
Folding of the two highly conserved motifs found in glycerophospholipid acyltransferases. This picture of the model of AGPAT2 showing the relationship between a conserved stretch of amino acids (Glu172, Gly173, Thr174, and Arg175) in AGPAT2 that have been proposed to be related to ligand binding. Red is the catalytic site H(X)4D; green is related to the cluster that binds the charged portion of the ligands, and the blue surface is the hydrophobic tunnel that accommodates the acyl chain. By manually docking C18:1-LPA, we observed that Arg175 in AGPAT2 is likely related to binding of the charged portions of LPA. The other conserved amino acids (Glu172, Gly173, and Thr174) in this region are behind the catalytic residues away from ligand docking. The highly conserved Gly173 in AGPAT2 can be involved in the plasticity of the active site during catalysis.