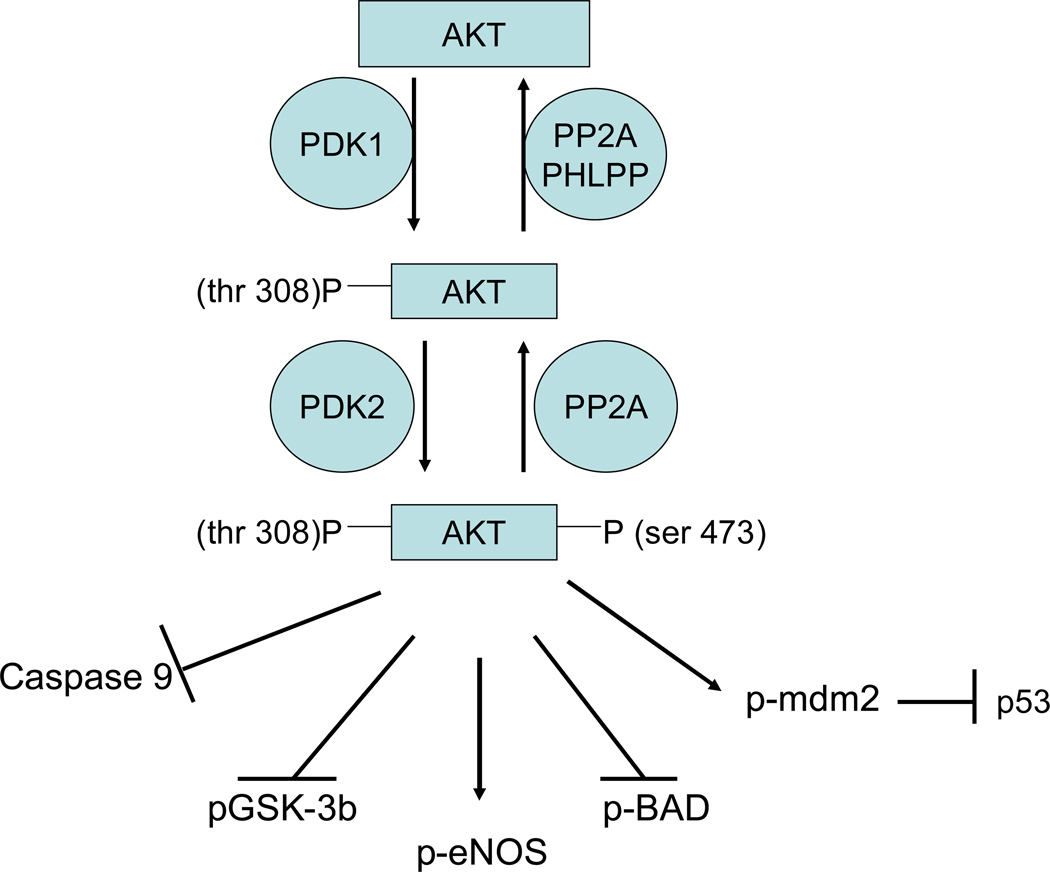
Figure 6.
AKT signaling. Akt is activated by phosphorylation of thr 308 by PDK1 and by phosphorylation of ser 473 by a poorly defined kinase. Phosphorylation of Akt at thr 308 is mediated by binding of PI3K generated lipid (PIP3) to the pleckstrin homology domain of Akt thereby facilitating translocation and association of Akt with PDK1. PIP3 binding to Akt also exposes the PDK1 phosphorylation site thus facilitating phosphorylation of Akt (188). A large number of kinases have been proposed to phosphorylate ser 473, including Akt autophosphorylation, PDK1, ILK1, MAPKAPK2, PKCbII, PIKK, ATM, DNA-PK, and TORC2 (see (68)). Increasing data seem to suggest a role for TORC2 in phosphorylating ser 473 on Akt (116). Akt can also be regulated by dephosphorylation, and PP2a (202) and PHLPP (33) have been reported to be important phosphatases regulating Akt phosphorylation.