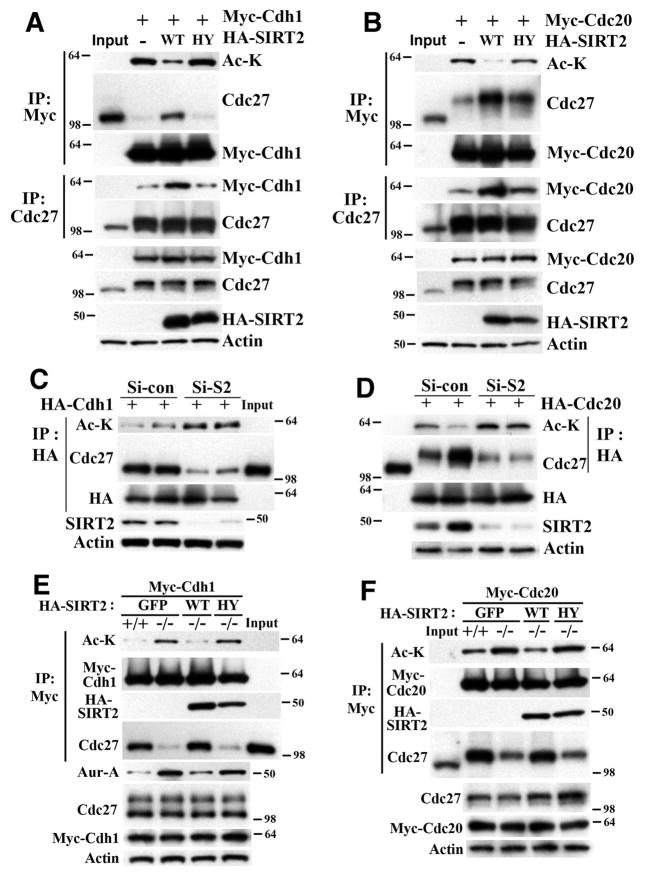
Figure 5. SIRT2 Regulates the Function of CDH1 and CDC20 through Deacetylation.
(A) Overexpression of WT but not deacetylase mutant (HY) SIRT2 decreases CDH1 acetylation and increases its interaction with CDC27 in HeLa cells. Ac-K: pan-acetyl lysine antibody.
(B) Overexpression of WT but not deacetylase mutant (HY) SIRT2 decreases CDC20 acetylation and increases its interaction with phospho-CDC27 in HeLa cells.
(C–D) Knockdown of SIRT2 by siRNA increases acetylation of CDH1 (C) and CDC20 (D) and decreases interaction with CDC27 or with phospho-CDC27, respectively, in HeLa cells. Si-S2 (siRNA against Sirt2), Si-Con (scramble siRNA).
(E) SIRT2 deficiency increases acetylation of CDH1 and decreases its interaction with CDC27, which is accompanied by an increase in Aurora-A in immortalized Sirt2 +/+ and −/− MEF cells.
(F) SIRT2 deficiency increases acetylation of CDC20 and decreases its interaction with CDC27 in immortalized SIRT2 WT and KO MEF cells. In all above panels, 24 hours after transfection, cells were synchronized by double thymidine block (HeLa cells) or serum starvation for 48 hr (MEF cells), released into regular media for 12 hr or 18 hr, respectively, Input: lysates from non-transfected and unsynchronized cells (5% in relation to IP samples) were used as control to distinguish phospho-CDC27 (migration slower) and non-phospho-CDC27.
(See also Figure S5)