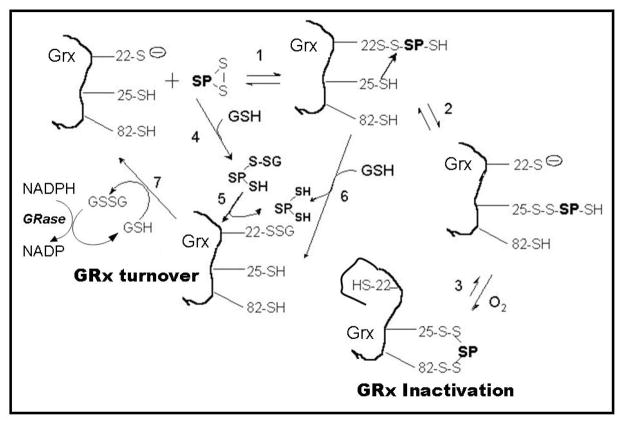
Figure 11. Mechanistic models of sporidesmin as a substrate and inactivator of GRx1.
This scheme shows two alternative explanations for sporidesmin as a substrate of GRx1: (a) steps 4, 5, 7 depict an initial non-enzymatic formation of glutathionyl mixed disulfide of sporidesmin, sporidesmin-S-SG, which is then reduced by GSH in a GRx1 catalyzed reaction to form reduced sporidesmin. This postulated mechanism is consistent with the two-substrate kinetics (Figure 1) and previous reports of the substrate specificity of GRx1 and the mode of substrate activity of non-GSH containing disulfides (1,2,4,5). (b) Steps 1, 6, 7 depict the direct reaction of sporidesmin with GRx1 to form GRx1 sporidesmin mixed disulfide (GRx1-S-S-sporidesmin). GSH then displaces sporidesmin from this adduct to form GRx1-S-SG which can enter the normal catalytic cycle of GRx1. (c) When GSH is scarce or absent the GRx1-S-S-sporidesmin complex goes on to an irreversibly inactivated form according to steps 1, 2, 3 involving molecular oxygen.