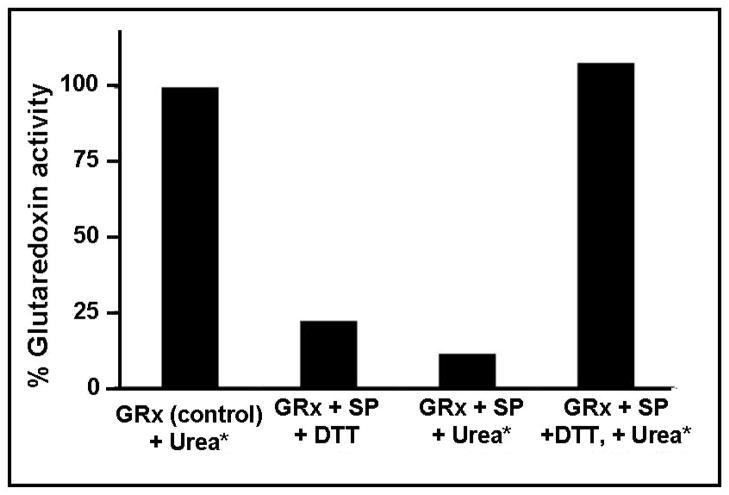
Figure 6. Requirements for reactivation of sporidesmin-inactivated GRx1.
GRx1 (100 μM) was treated either with 1 mM sporidesmin or 5 % ethanol at 30 °C, 0.1 M K phosphate, pH 7.5. Enzyme activity was measured after incubation for 15 min. The sporidesmin-treated sample was approximately 90% inactivated. The control was treated with 8 M urea plus 50 mM DTT. The inactivated GRx1 was divided into two aliquots and treated either with either 8 M urea or 50 mM DTT and 8 M urea. The samples (including urea plus DTT treated control) were each loaded on a gel filtration column and separated from excess small molecules. The GRx1 protein was pooled and analyzed for activity and protein content. The results shown in the figure are for a single experiment, after conditions were optimized. The recovery of protein after size exclusion chromatography was 55–60% for all three samples. GRx1 inactivated with sporidesmin and exposed to urea alone or DTT alone showed no reactivation, while treatment with sporidesmin then urea plus DTT gave 60% recovery of activity, i.e. full recovery of GRx activity when normalized to 55–60% protein recovery.