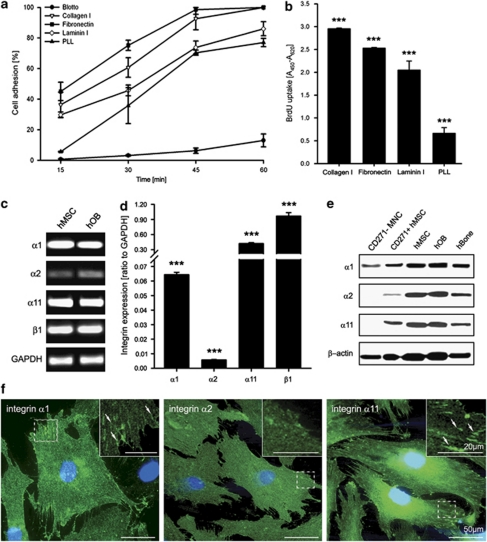
Figure 1.
hMSC adhesion and proliferation on ECM proteins, and integrin expression profile. (a) Quantitative adhesion assay on collagen I, fibronectin, laminin I, PLL (poly--lysine) and blotto (5% milk/PBS). The highest hMSC adhesion was toward fibronectin and collagen I. The data represent two independent experiments. (b) Proliferation analysis. Cells were cultured for 24 h in the presence of 10 μM BrdU. The highest BrdU uptake was detected when hMSC were grown on collagen I. Three independent experiments, each consisting of triplicates, were performed (n=9; ***P<0.001; one-way ANOVA). (c) Semi-quantitative PCR analysis of the basal expression of α1, α2, α11 and β1 integrin in hMSC and hOB. Shown is a representative experiment of three independent repeats. (d) Quantitative PCR analysis of collagen I-binding integrin expression revealed that α11 is the most strongly expressed integrin subunit in hMSC cultivated on collagen I. Data consist of three independent quantitative measurements with three donors (n=9; ***P<0.001; one-way ANOVA). (e) Representative western blots for integrin α1, α2 and α11 in freshly isolated CD271-MNC and CD271+hMSC, cultivated hMSC and hOB, and hBone protein extracts confirmed integrin protein expression in vivo and in vitro. (f) Immunoflourescent stainings of α1, α2 and α11 integrins of hMSC cultured on collagen I. Integrin α11 showed the most pronounced focal adhesions (indicated by arrows). The staining was reproduced twice, bar 50 μm and in the inset 20 μm