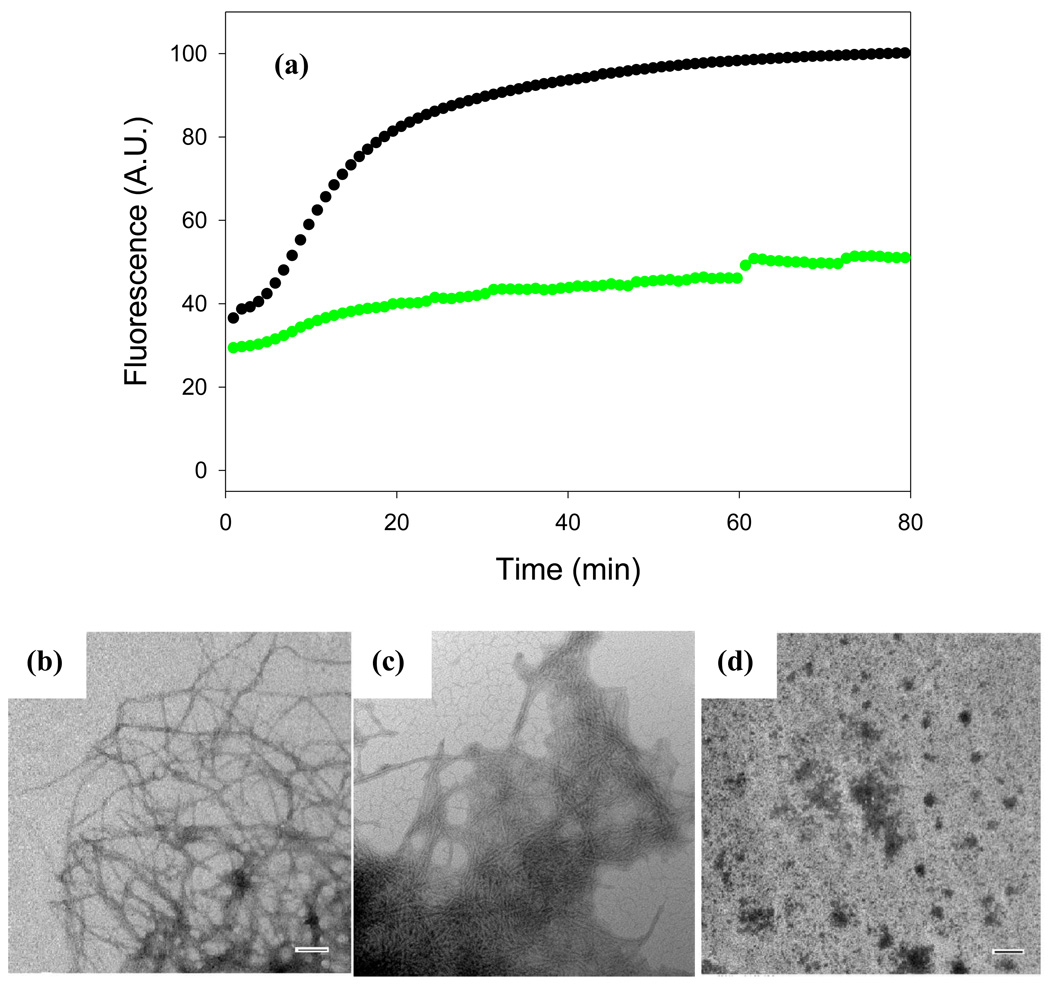
Figure 3. Acid fuchsin inhibits amyloid formation by a mixture of proIAPP1–48 and heparan sulfate.
(a) Time course of amyloid fibril formation monitored by thioflavin-T binding: proIAPP1–48 with heparan sulfate in the absence of inhibitor (black); a 1:1 mixture of acid fuchsin with proIAPP1–48/heparan sulfate (green). (b) TEM image of the amyloid fibrils formed by the proIAPP1–48/heparan sulfate mixture in the absence of acid fuchsin. Samples were removed 80 minutes after the start of the reaction. (c) TEM image of a sample of the 1:1 mixture of acid fuchsin with proIAPP1–48/heparan sulfate collected at 80 minutes after the initiation of the reaction. (d) TEM image of a sample of the 10:1 mixture of acid fuchsin with proIAPP1–48/heparan sulfate collected at 80 minutes after the initiation of the reaction. Scale bars represent 100 nm. The same solutions were used for the kinetic studies and for the TEM samples. The pH of the solutions was 7.4. The solutions contained 2% HFIP by volume, 20mM Tris-HCl, 25µM thioflavin-T, 32µM peptide and were continually stirred at 25°C.