Abstract
Sigma receptor (σR) antagonists attenuate many behavioral effects of cocaine but typically not its reinforcing effects in self-administration procedures. However, the σR antagonist rimcazole and its N-propylphenyl analogs, [3-(cis-3,5-dimethyl-4-[3-phenylpropyl]-1-piperazinyl)-propyl]diphenylamine hydrochloride (SH 3-24) and 9-[3-(cis-3,5-dimethyl-4-[3-phenylpropyl]-1-piperazinyl)-propyl]carbazole hydrobromide (SH 3-28), dose-dependently decreased the maximal rates of cocaine self-administration without affecting comparable responding maintained by food reinforcement. In contrast, a variety of σR antagonists [N-phenethylpiperidine oxalate (AC927), N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide (BD 1008), N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino) ethylamine dihydrobromide (BD 1047), N-[2-(3,4-dichlorophenyl) ethyl]-4-methylpiperazine dihydrochloride (BD 1063), and N,N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]-ethylamine monohydrochloride (NE-100)] had no effect on cocaine self-administration across the range of doses that decreased rates of food-maintained responding. Rimcazole analogs differed from selective σR antagonists in their dual affinities for σRs and the dopamine transporter (DAT) assessed with radioligand binding. Selective DAT inhibitors and σR antagonists were studied alone and in combination on cocaine self-administration to determine whether actions at both σRs and the DAT were sufficient to reproduce the effects of rimcazole analogs. Typical DAT inhibitors [2β-carbomethoxy-3β-(4-fluorophenyl)tropane (WIN 35,428), methylphenidate, and nomifensine] dose-dependently shifted the cocaine dose-effect curve leftward. Combinations of DAT inhibitor and σR antagonist doses that were behaviorally inactive alone decreased cocaine self-administration without effects on food-maintained responding. In addition, whereas the DAT inhibitors were self-administered at rates similar to those of cocaine, neither rimcazole analogs nor typical σR antagonists (NE-100 and AC927) maintained responding above control levels across a wide range of doses. These findings suggest that the unique effects of rimcazole analogs are due to dual actions at the DAT and σRs and that a combined target approach may have utility in development of medical treatments for cocaine abuse.
Introduction
Several studies have demonstrated that σ receptor (σR) antagonists block some behavioral effects of cocaine. For example, pretreatment with σR antagonists, α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazine-butanol (BMY 14802) or 9-[3-(cis-3,5-dimethyl-1-piperazinyl)propyl]-9H-carbazole dihydrochloride (rimcazole), antagonized locomotor stimulatory effects of cocaine in mice (Menkel et al., 1991). In addition, several σR antagonists significantly attenuated the development of stimulant-induced locomotor sensitization (Ujike et al., 1992; Witkin et al., 1993). Furthermore, pretreatment with a variety of σR antagonists reduces the acute toxic effects of cocaine, including convulsions and lethality (Maurice and Su, 2009; for reviews, see Matsumoto, 2009).
Despite the blockade of locomotor stimulation and acute toxic effects of cocaine by a variety of σR antagonists, studies on the blockade of effects of cocaine related to reinforcement have reported inconsistent outcomes. Pretreatments with σR antagonists N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide (BD 1047) or N,N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]-ethylamine monohydrochloride (NE-100) decreased cocaine-induced place conditioning in mice (Romieu et al., 2002, 2004). However, an initial report showed that a selected dose of BD 1047 on cocaine self-administration in rats was inactive (Martin-Fardon et al., 2007). A subsequent study that examined a wide range of doses of BD 1047 and its analogs (BD 1008 and BD 1063) similarly found a lack of effects on self-administration of a broad range of cocaine doses (Hiranita et al., 2010).
The mechanism by which σR antagonists block some of the effects of cocaine has not been determined. A number of studies have suggested interactions between σR and dopamine systems in which cocaine appears to act predominantly to produce its behavioral effects. For example, Wachtel and White (1988) showed that BMY 14802 reversed the suppressant effects of apomorphine on the firing of both A9 and A10 dopamine neurons. Furthermore, Gonzalez-Alvear and Werling (1994) showed a σR-mediated modulation of dopamine release, and Su and colleagues (e.g., Hayashi et al., 2000) showed that σRs are linked to intracellular calcium signaling mechanisms that may alter the short-term or long-term effects of cocaine (Su and Hayashi, 2001). With an extensive array of intracellular functions of σRs, there are many potential mechanisms that may contribute to the interactions of stimulants and σR ligands (Katz et al., 2011).
Matsumoto et al. (2001) suggested that the interaction between cocaine and σR ligands is a competitive antagonism of effects of cocaine mediated by σRs. On the other hand, Izenwasser et al. (1993) found that several σR ligands, including rimcazole, inhibited dopamine uptake and had micromolar affinity for the DAT. Thus, some σR ligands may alter the effects of cocaine through an action at the DAT. Consistent with this interpretation, a recent finding (Loland et al., 2008) showed that rimcazole analogs bind to the DAT in a manner favoring a DAT conformation that renders it less accessible to the extracellular space. In contrast, cocaine analogs bind to the DAT in a manner that favors an outward-facing conformation (Reith et al., 2001; Loland et al., 2008).
Rimcazole and two of its analogs, [3-(cis-3,5-dimethyl-4-[3-phenylpropyl]-1-piperazinyl)-propyl]diphenylamine hydrochloride (SH 3-24) and 9-[3-(cis-3,5-dimethyl-4-[3-phenylpropyl]-1-piperazinyl)-propyl]carbazole hydrobromide (SH 3-28) (Husbands et al., 1999), were reported to block the locomotor stimulant effects of cocaine at doses that were themselves behaviorally inactive (Menkel et al., 1991; Katz et al., 2003). These findings coupled with those of Loland et al. (2008) suggest that rimcazole and its analogs may promote a conformational change in the DAT that disfavors cocaine binding and contributes to a distinctive behavioral profile. Therefore, the interaction of rimcazole and its analogs with cocaine may differ from that of other σR antagonists because of their dual actions. As such, rimcazole analogs, in contrast to selective σR antagonists, may more effectively block cocaine self-administration. Thus, the present study assessed the activity of rimcazole and two of its analogs in rats self-administering cocaine and their own abuse liability. Each of the compounds decreased rates of responding maintained by cocaine at doses that had no effects on responding maintained by food reinforcement. Because the compounds have affinity for the DAT as well as σRs, we subsequently assessed whether dual actions at these sites contributes to the blockade of cocaine self-administration through combinations of compounds selective for these sites.
Materials and Methods
Subjects.
Thirty-eight male Sprague-Dawley rats (weighing approximately 300 g at the start of the study), obtained from Charles River Laboratories (Wilmington, MA), served as subjects after acclimation to the laboratory for at least 1 week. Some of these subjects had been used previously in a study using similar behavioral and pharmacological procedures (Hiranita et al., 2010). Food (Scored Bacon Lover Treats; Bio-Serv, Frenchtown, NJ) and tap water were available in their home cages. After acclimation, weights of rats were maintained at approximately 320 g by adjusting their daily food ration. The animal housing room was temperature- and humidity-controlled and maintained on a 12-h light/dark cycle with lights on at 7:00 AM. Care of the subjects was in accordance with the guidelines of the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program Animal Care and Use Program, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Apparatus.
Experimental sessions were conducted with subjects placed in operant-conditioning chambers (modified ENV-008CT; Med Associates, St. Albans, VT) that measured 25.5 cm × 32.0 cm × 25.0 cm and were enclosed within sound-attenuating cubicles equipped with a fan for ventilation and white noise to mask extraneous sounds. On the front wall of each chamber were two response levers, 5.0 cm from the midline and 4.0 cm above the grid floor. A downward displacement of a lever with a force approximating 20 g defined a response, which always activated a relay mounted behind the front wall of the chamber producing an audible “feedback” click. Three light-emitting diodes (LEDs) were located in a row above each lever. A receptacle for the delivery of food pellets was mounted behind a 5.0 × 5.0 cm opening in the front wall midline between the two levers and 2.0 cm above the floor. When called for, a pellet dispenser (ENV-203; Med Associates) delivered 45-mg food pellets to the receptacle. A syringe driver (model 22; Harvard Apparatus, Holliston, MA) placed above each chamber delivered injections of specified volumes and durations from a 10-ml syringe. The syringe was connected by Tygon tubing to a single-channel fluid swivel (375 Series Single Channel Swivels; Instech Laboratories, Inc., Plymouth Meeting, PA), which was mounted on a balance arm above the chamber. Tygon tubing from the swivel to the subject's catheter was protected by a surrounding metal spring and completed the connection to the subject.
Procedures.
Subjects were placed in chambers during experimental sessions that were conducted daily, 7 days per week. During sessions, subjects were trained with food reinforcement (45-mg food pellets; Bio-Serv) to press the right lever and were subsequently trained under a fixed-ratio 5 response schedule of reinforcement (each fifth response produced a food pellet). Food deliveries were followed by a 20-s timeout (TO) period during which all lights were off and responses had no scheduled consequences other than the feedback click. During this training, sessions lasted for 20 min or until 30 food pellets were delivered.
After subjects were responding at a rate sufficiently high that they obtained 30 food pellets within each of three consecutive sessions, they were divided into two groups. One group continued with food reinforcement, whereas subjects in the other group were surgically implanted in the right or left external jugular vein with a chronic indwelling catheter that exited at the midscapular region of the animal's back. Catheter implantation was performed under anesthesia (60.0 mg/kg i.p. ketamine and 12.0 mg/kg i.p. xylazine). Catheters were infused daily with 0.1 ml of a sterile saline solution containing heparin (30.0 IU/ml) and penicillin G potassium (250,000 IU/ml) to minimize the likelihood of infection and the formation of clots or fibroids. All animals were allowed to recover from surgery for approximately 7 days before cocaine self-administration studies were initiated.
Cocaine self-administration sessions were conducted in 2-h daily sessions until the response rates and patterns of responding showed no substantial session-to-session trends. During these sessions, the LEDs above the right lever were illuminated when cocaine injections were available. Completion of five responses turned off the LEDs and activated the infusion pump, delivering a dose of 1.0 mg/kg. A 20-s TO, during which LEDs were off and responses produced only feedback clicks, started with the injection. After the timeout, the LEDs were illuminated, and responding again had scheduled consequences. Once rates of responding maintained by cocaine were stable across sessions, the session was divided into five 20-min components, each preceded by a 2-min TO. This arrangement allowed the assessment of a different cocaine dose within each component. By adjusting infusion volumes and durations, the cocaine dose per injection was incremented in the five sequential components in an ascending order as follows: no injection [also referred to as extinction (EXT) because responses had no scheduled consequences other than turning off the LEDs for 20 s] and 0.03, 0.10, 0.32, and 1.0 mg/kg per injection (inj). Infusion volumes and durations were, respectively, 0, 5.6, 18.0, 56.0, and 180 μl and 0, 0.32, 1.0, 3.2, and 10.0 s, based on a body weight of 0.32 kg. A response-independent “sample” injection of cocaine at the corresponding dose was administered immediately before each component.
Training continued until 1) at least 5.0 mg/kg cocaine was self-administered within a session with less than 20% variation in the total number of cocaine injections compared with that in the previous session; 2) the dose of cocaine that maintained the maximal response rates varied by no more than 1/2 log unit over two consecutive test sessions; and 3) maximal response rates were at least 5-fold higher than response rates maintained during EXT. The effects of substitution of other drugs for cocaine or presession treatments on cocaine self-administration were separated by a minimum of 72 h and were conducted only if performances met the training criteria. All of the tests were conducted with a mixed order of drugs and doses.
The schedule of food reinforcement was also modified as in the present studies of cocaine self-administration, with five sequential 20-min components, each preceded by a 2-min TO. The first of the five components was EXT (no food available), with a fixed ratio 5 schedule of food delivery in effect in the subsequent four components. Subjects were given their daily (∼15 g) ration of food (Harlan Rodent Chow) 60 min before sessions, so that their response rates approached those maintained by cocaine.
Once performances were stable across successive sessions, the effects of substitutions for cocaine of saline, dopamine uptake inhibitors, rimcazole analogs, and selective σR antagonists were assessed, with a minimum of 72 h between treatments. The dopamine uptake inhibitors studied were 2β-carbomethoxy-3β-(4-fluorophenyl)tropane (WIN 35,428) (0.0032–0.1 mg/kg per inj i.v.), methylphenidate (0.032–1.0 mg/kg per inj i.v.), and nomifensine (0.01–0.32 mg/kg per inj i.v.). Substitutions of rimcazole (0.1–3.2 mg/kg per inj i.v.) and its analogs, SH 3-24 (0.032–1.0 mg/kg per inj i.v.) and SH 3-28 (0.1–3.2 mg/kg per inj i.v.), were compared with those for the σR antagonists, N-phenethylpiperidine oxalate (AC927) and NE-100 (0.032–1.0 mg/kg per inj i.v. each). Subsequently, the effects of presession i.p. injections of the above drugs on the response rates maintained by cocaine injection or food presentation were assessed. Finally, the effects of combined presession intraperitoneal injection of the dopamine uptake inhibitors and the σR antagonists, BD 1008, BD 1047, or BD 1063, on the response rates maintained by cocaine injection or food presentation were also assessed. The dose ranges examined in these experiments are shown in the figures.
Dopamine Transporter Binding Assay.
Frozen brains from male Sprague-Dawley rats weighing 200 to 225 g (Taconic Laboratories, Germantown, NY) were thawed on ice, and the striatum was dissected, homogenized in ice-cold modified sucrose-phosphate buffer (0.32 M sucrose, 7.74 mM Na2HPO4, and 2.26 mM NaH2PO4, pH adjusted to 7.4) using a Brinkmann Polytron (setting 6 for 20 s), and centrifuged at 50,000g for 10 min at 4°C. The resulting pellet was resuspended in buffer, centrifuged, and suspended in buffer again to a concentration of 10 mg/ml original wet weight (OWW).
Ligand binding experiments were conducted in assay tubes containing 0.5 ml of sucrose-phosphate buffer. Each tube contained 0.5 nM [3H]WIN 35,428 (specific activity 84 Ci/mmol; PerkinElmer Life and Analytical Sciences, Waltham, MA) and 1.0 mg of striatal tissue, OWW. The reaction was started with the addition of tissue, and the tubes were incubated for 120 min on ice. Nonspecific binding was determined using 0.1 mM cocaine HCl (Sigma-Aldrich, St. Louis, MO).
σR Binding.
Frozen whole guinea pig brains (minus cerebellum) were thawed on ice, weighed, and homogenized (with a glass and Teflon homogenizer) in 10 mM Tris-HCl with 0.32 M sucrose, pH 7.4 (10 ml/g tissue). Guinea pig brain was used because of the relatively higher density of those receptors in that tissue compared with rat tissue (Tam, 1983). The homogenate was centrifuged at 1000g for 10 min at 4°C. The supernatant was collected into a clean centrifuge tube, and the remaining pellet was resuspended by vortex in 10 ml of buffer (tissue) and centrifuged again at 50,000g for 15 min at 4°C. The resulting pellet was resuspended in experimental buffer to 80 mg/ml, OWW.
Ligand binding experiments were conducted in polypropylene assay tubes containing 0.5 ml of 50 mM Tris-HCl buffer, pH 8.0. For σ1R binding, each tube contained 3 nM [3H](+)-pentazocine (PerkinElmer Life and Analytical Sciences) and 8.0 mg of tissue, OWW. Nonspecific binding was determined using 10 μM haloperidol. For σ2R binding, each tube contained 3 nM [3H]DTG (PerkinElmer Life and Analytical Sciences), 200 nM (+)-pentazocine, and 8.0 mg of tissue, OWW. Nonspecific binding was determined using 100 μM haloperidol. The reaction was started with the addition of tissue, and the tubes were incubated for 120 min at room temperature.
Incubations for all binding assays were terminated by rapid filtration through Whatman GF/B filters, presoaked in polyethylenimine, using a Brandel R48 filtering manifold (Brandel Inc., Gaithersburg, MD). The filters were washed twice with 5 ml of ice-cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0 ml) was added, and the vials were counted the next day using a Beckman 6000 liquid scintillation counter (Beckman Coulter, Fullerton, CA) at 50% efficiency. Assays were typically conducted in at least three independent experiments, each performed in triplicate.
Drugs.
The drugs used in the present study were as follows: (−)-cocaine hydrochloride (Sigma-Aldrich), WIN 35,428 (NIDA, Drug Supply Program), methylphenidate (NIDA), nomifensine (NIDA), rimcazole (Sigma-Aldrich), AC927 (gift from Dr. Andrew Coop, University of Maryland School of Pharmacy, Baltimore, MD), NE-100 (gift from Dr. Tsung-Ping Su, National Institute on Drug Abuse, Baltimore, MD), BD 1008 (Tocris Bioscience, Ellisville, MO), BD 1047 (Tocris Bioscience), and BD 1063 (Tocris Bioscience). Rimcazole analogs were synthesized in the Medicinal Chemistry Section, NIDA Intramural Research Program (Husbands et al., 1999). Self-administration of the test drugs was assessed with intravenous delivery of injections, whereas drug pretreatments were administered intraperitoneally. All drugs were administered at 5 min before sessions with the exception of BD 1047, which was administered at 15 min before sessions. All drug solutions were prepared fresh daily in 0.9% NaCl, with the exception of SH 3-28 (initially dissolved in 0.16% tartaric acid and with final volumes achieved by addition of sterile water). Pretreatment times and doses of drugs used in the present study were chosen on the basis of published data (Katz et al., 2003; Hiranita et al., 2010) or preliminary data obtained in this laboratory.
Data Analysis.
For the radioligand binding assays, the IC50 values from the displacement data were computed using a nonlinear, least-squares regression analysis (GraphPad Prism, GraphPad Software Inc., San Diego, CA). Inhibition constants (Ki values) were calculated using the concentration of radioligand used in the assay, and the historical value for the Kd value of the radioligand determined in this laboratory.
For the behavioral data, response rates were determined by dividing responses by elapsed time in each component, excluding the timeouts that followed injections or food presentations. Average values across six subjects (with S.E.M.) are presented below. To determine whether there was a difference in effects of cocaine compared with saline self-administration, a two-way, repeated-measures analysis of variance (ANOVA) was used (factors were component and substance injected: cocaine or saline). A one-way, repeated-measures ANOVA was used to assess the effects of successive components in the substitution for cocaine of the test drugs. A two-way (repeated) measures ANOVA was used to assess the effects of presession treatments of the test drugs on cocaine self-administration and for the comparison of effects of drug pretreatments on responding maintained by cocaine injection (0.32 mg/kg per inj) or food reinforcement. For studies of prior drug treatments on self-administration of cocaine, a post hoc Bonferroni t test was used for pairwise comparisons. For assessments of the selectivity of drug pretreatments on responding maintained by cocaine injection or food presentation, the effects on responding during the fourth component (in which maximal response rates were maintained by cocaine injection under control conditions) were analyzed by two-way (repeated) measures ANOVA, with a post hoc Bonferroni t test used for pairwise comparisons.
Results
Radioligand Binding Assays.
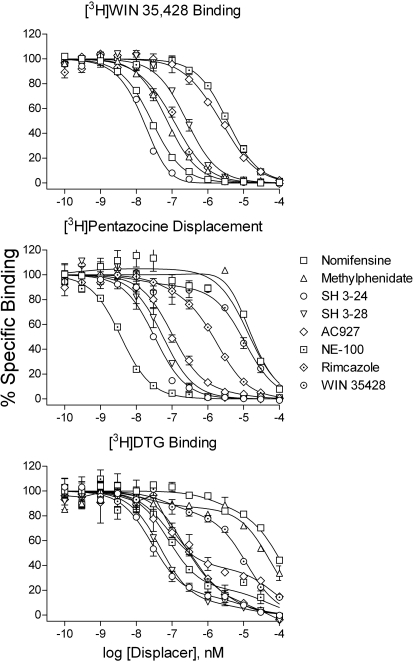
Consistent with previous reports (e.g., Izenwasser et al., 1993; Husbands et al., 1999), rimcazole and its N-propylphenyl analogs displaced [3H]WIN 35,428 with relatively high affinity. The affinity at the DAT of the diphenylamine, SH 3-24, was comparable to those of the DAT inhibitors, WIN 35,428, nomifensine, and methylphenidate (Fig. 1; Table 1) and higher than that for rimcazole or SH 3-28. In contrast, the Ki values at the DAT for the selective σR antagonists (AC927, BD 1008, BD 1047, NE-100, and BD 1063) were at a minimum 10-fold lower than that for SH 3-28 and approximately 160-fold lower than that for SH 3-24 (Table 1).
Fig. 1.
Displacement of radioligands for the DAT and σRs by various ligands for the binding sites. Ordinates, percentage of specific radiotracer bound to membrane preparations as described under Materials and Methods; abscissae, concentration of each competing compound. Top panel, binding to the DAT as labeled with [3H]WIN 35,428. Middle panel, binding to σ1Rs as labeled with [3H]pentazocine. Bottom panel, binding to σ2Rs as labeled with [3H]DTG. The curves represent the results of a single experiment with vertical bars representing S.E.M.s from averages of results from three samples. The results were selected from at least three replications as representative of the binding parameters resulting from global modeling of all of the data.
TABLE 1.
Inhibition by various compounds of specific binding to the DAT, σ1, or σ2 receptors
The values listed are Ki values ± S.E.M. (95% confidence limits), with the exception of the value for WIN 35,428 at the DAT, which is a Kd value obtained from a homologous competition study. See Materials and Methods for details of the assay procedures and derivation of Ki values.
| Compound |
Ki Value |
||
|---|---|---|---|
| DAT | σ1 | σ2 | |
| mM | |||
| WIN 35,428 | 5.24 (4.92–5.57)a | 5700 (4060–8020) | 4160 (3120–5550) |
| SH 3-24 | 12.2 (10.8–13.8) | 22.9 (18.5–28.2) | 20.0 (15.7–25.6) |
| Nomifensine | 21.0 (18.9–23.3) | 8240 (5360–12,700) | 65,200 (54,300–78,300) |
| Methylphenidate | 65.8 (61.2–70.8) | 6780 (4520–10,200) | 37,400 (21,200–66,100) |
| Cocaineb | 76.6 (72.6–80.5) | 5190 (3800–7060) | 19,300 (16,000–23,300) |
| Rimcazole | 96.6 (77.3–121) | 883 (661–1180) | 238 (171–329) |
| SH 3-28 | 188 (166–213) | 19.0 (15.3–23.6) | 47.2 (40.4–55.2) |
| AC927 | 1,930 (1610–2320) | 53.1 (45.6–61.8) | 78.9 (48.2–129) |
| BD 1008b | 2,510 (2250–2790) | 2.13 (1.77–2.56) | 16.6 (13.0–21.1) |
| BD 1047b | 3,220 (2820–3670) | 3.13 (2.68–3.65) | 47.5 (36.7–61.4) |
| NE-100 | 3,590 (3,210–4,000) | 2.48 (2.13–2.88) | 121 (91.9–159) |
| BD 1063b | 8,020 (7100–9060) | 8.81 (7.15–10.9) | 625 (447–877) |
The value for affinity of WIN 35,428 at the DAT is a Kd value obtained from a homologous competition study.
The values for these compounds are those reported in the literature by Garcés-Ramírez et al. (2010) using methods identical to those in the present study. For the [3H]DTG assay, the data typically modeled better for two than one binding site, and the Ki values for the higher affinity site are displayed in the table. The DTG high-affinity site is the site recognized as the σ2 receptor, whereas the low-affinity site is currently not identified. Values for the low-affinity DTG site (95% confidence limits) were as follows: SH 3-24, 12,700 (1300–124,000) nM; rimcazole, 25,900 (3620–185,000) nM; AC927, 55,200 (6860–444,000) nM; BD 1008, 20,500 (9640–43,500) nM; BD 1047, 55,300 (25,000–122,000) nM; and BD 1063, 53,700 (16,500–174,000) nM.
Rimcazole had greater affinity than the DAT inhibitors at σ1 receptors, as labeled with [3H]pentazocine (Fig. 1; Table 1). However, rimcazole had the lowest affinity among recognized σR ligands, and NE-100 was among those with the highest affinity for σ1Rs and most selective for σ1 over σ2 receptors, although that selectivity was only approximately 50-fold (Table 1).
A previous study using the same methods showed data from [3H]DTG binding, as well as its displacement by several drugs, to be better fit by a two- than a one-site model (Garcés-Ramírez et al., 2011). Similar results were obtained in the present study (Fig. 1). Because the Ki values for known compounds at the higher-affinity site corresponded closely with published literature on σ2R binding, all the data were fit to a two-site model and the values for the high-affinity site are included in Table 1, with those for the lower-affinity site in its footnote. Most of the σR antagonists had higher affinity for the σ1 than σ2 receptor, with the most selective being BD 1063. In contrast, rimcazole, although having low affinity compared with its analogs, preferentially bound σ2Rs compared with σ1Rs. AC927 had a slightly higher affinity at the σ1R compared with the σ2R. Finally, Ki value of the DAT inhibitors at either of the σRs was between 2 and 4 orders of magnitude greater than their Ki values at the DAT (Fig. 1; Table 1).
Drug Self-Administration Procedures.
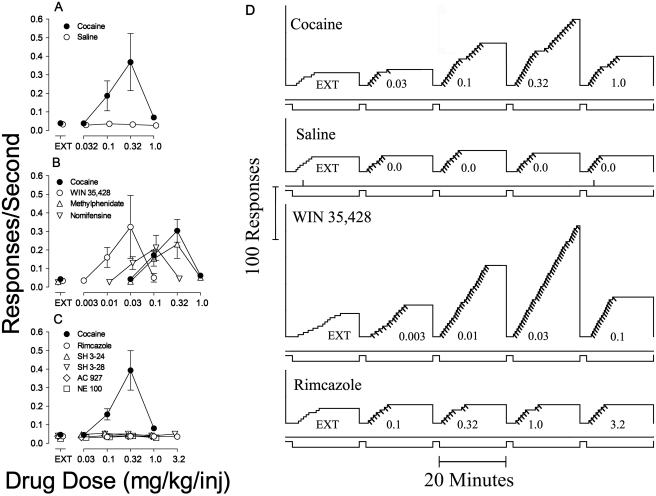
The average response rates maintained by cocaine were a bell-shaped function of dose, with a maximum of 0.37 ± 0.15 response/s when 0.32 mg/kg per inj maintained responding (during the fourth component). This response rate was approximately 10-fold greater than the 0.04 response/s occurring during EXT (the first component in Fig. 2A, ● above EXT) or those maintained by saline (Fig. 2A, ○). One-way repeated-measures ANOVA indicated a significant effect of cocaine dose on response rate (p < 0.001). Subsequent replications with cocaine (Fig. 2, B and C) showed similar results. Cumulative records of performances show low rates of responding when responses did not produce injections (Fig. 2D, top panel, EXT, first component). When responses produced cocaine injections, brief pauses were followed by a sequence of five responses in rapid sequence until the injection was delivered (Fig. 2D, top panel, second to fifth components). The highest rate of responding was obtained in the fourth component, in which injections of 0.32 mg/kg per inj were available. As was typically observed, the records in Fig. 2 show little or no response on the inactive lever (perpendicular marks on the lines below the cumulative curve) or during the 2-min TO periods between successive components (lowest line displaced upward). Saline did not maintain rates of responding greater than those obtained in EXT (Fig. 2, A and D, second panel); one-way repeated-measures ANOVA indicated a nonsignificant effect of component number on response rate when saline injections were available (F = 0.495, p = 0.739).
Fig. 2.
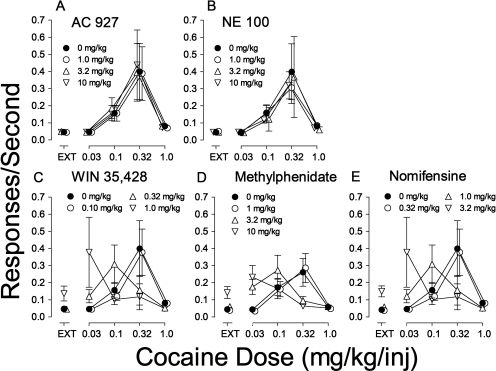
Substitution of saline, dopamine uptake inhibitors (WIN 35,428, methylphenidate, and nomifensine), rimcazole and its analogs (SH 3-24 and SH 3-28), and σR antagonists (AC927 and NE-100) in rats trained to self-administer cocaine. A–C, ordinates, responses per second; abscissae, dose of each substituted drug in milligrams per kilogram per injection. Each point represents the mean ± S.E.M. (n = 6–19). A, cocaine (●) and saline (○). B, cocaine replication (●), WIN 35,428 (○), methylphenidate (▵), and nomifensine (▿). C, cocaine replication (●), rimcazole (○), SH 3-24 (▵), SH 3-28 (▿), AC927 (♢), and NE-100 (□). D, representative cumulative records showing patterns of self-administration in real time maintained by intravenous cocaine injection under the fixed-ratio 5 response schedule, and those obtained when saline, WIN 35,428, or the σR antagonist, rimcazole, was substituted for cocaine. Ordinates, cumulative responses; abscissae, time. The five 20-min self-administration components of each session are indicated by the lower event line displaced down. The preceding 2-min TO periods are indicated by the lower event line displaced up. In the first component, each fifth response turned off the LEDs for 20 s but did not activate the infusion pump (EXT), whereas in subsequent components injections were also delivered with each fifth response (diagonal marks on the cumulative record) with doses (in milligrams per kilogram per injection) indicated. Vertical marks on the line below the cumulative curve indicate responses on the left (inactive) lever. The cumulative curve reset to the baseline at the end of the 20-min component.
The dopamine uptake inhibitor WIN 35,428 maintained responding that resembled that maintained by cocaine (Fig. 2, B and D, third panel). The highest rate of responding was maintained at a dose of 0.03 mg/kg per inj, with lower response rates at higher and lower doses (Fig. 2B, ○). The shape of the WIN 35,428 dose-effect curve and the maximal response rates maintained were comparable to those for cocaine; WIN 35,428 was approximately 10-fold more potent than cocaine (Fig. 2B). A one-way repeated-measures ANOVA confirmed the statistical significance of the effects of the WIN 35,428 component on response rates (F = 3.37, p = 0.025).
Similar to WIN 35,428, the dopamine uptake inhibitors, methylphenidate and nomifensine, maintained responding that resembled that maintained by cocaine with regard to maximal response rates and the shape of the dose-effect curves (Fig. 2B). The highest rates of responding maintained by methylphenidate and nomifensine were at doses of 0.32 and 0.1 mg/kg per inj, respectively, with lower response rates at higher and lower doses (Fig. 2B, ▵ and ▿, respectively). Methylphenidate was equipotent to cocaine, whereas nomifensine was approximately 3-fold more potent than cocaine (Fig. 2B). Response rates maintained by methylphenidate and nomifensine (F ≥ 8.48, p < 0.001; one-way repeated-measures ANOVA) were significantly affected by dose.
In contrast to the effects of the dopamine uptake inhibitors, no dose of rimcazole (Fig. 2C and 2D, fourth panel) or of its analogs, SH 3-24 and SH 3-28, maintained response rates comparable to those maintained by cocaine (Fig. 2C). A one-way repeated-measures ANOVA indicated nonsignificant effects of rimcazole (F = 2.19, p = 0.107), although the effects of SH 3-24 (F = 4.54, p = 0.009) and SH 3-28 (F4,20 = 6.76, p = 0.001) doses were significant. As can be seen in Fig. 2C, the response rates maintained by either rimcazole analog were only marginally different from those maintained by vehicle and far below response rates maintained by cocaine or the other DAT inhibitors (Fig. 2B).
Similar to rimcazole and its analogs, no dose of either of the σR antagonists, AC927 and NE-100, maintained response rates comparable to those maintained by cocaine (Fig. 2C). Nonetheless, one-way-repeated-measures ANOVA indicated significant effects of dose of AC927 (F4,20 = 9.46, p < 0.001) and NE-100 (F = 71.0, p < 0.001). As with rimcazole analogs, none of the significant increases in response rates with these σR antagonists approached those maintained by cocaine or the other DAT inhibitors (Fig. 2C).
Pretreatment with Rimcazole Analogs.
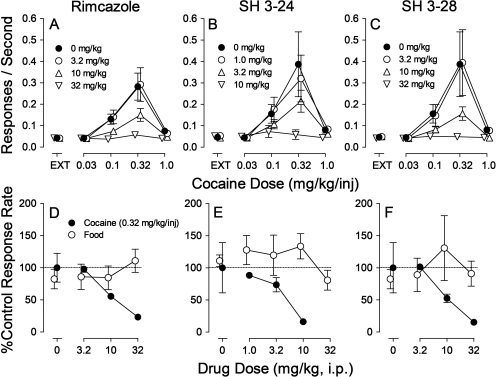
When administered before sessions, rimcazole dose-dependently decreased response rates maintained by cocaine (Fig. 3A). The decreases were expressed as a flattening of the bell-shaped cocaine dose-effect curve. At the highest dose of rimcazole, no dose of cocaine maintained responding at levels appreciably greater than those maintained in EXT (Fig. 3A; Fig. 4, compare first and second panels). Two-way repeated-measures ANOVA of the effects of rimcazole on response rates indicated a significant effect of cocaine dose (F4,60 = 16.5, p < 0.001), presession rimcazole treatment (F3,60 = 14.2, p < 0.001), and the interaction of the two (F12,60 = 10.6, p < 0.001).
Fig. 3.
Effects of presession treatments with rimcazole and its analogs on responding maintained by cocaine injection or food presentation. Each point represents the mean ± S.E.M. (n = 6). Rimcazole and its analogs were administered intraperitoneally at 5 min before sessions. A–C, effects of presession treatments with rimcazole and its analogs on cocaine self-administration. Ordinates, responses per second; abscissae, cocaine injection dose in milligrams per kilogram. A, effects of rimcazole (3.2, 10, and 32 mg/kg) on cocaine self-administration. B, effects of SH 3-24 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration. C, effects of SH 3-28 (3.2, 10, and 32 mg/kg) on cocaine self-administration. D–F, effects of presession treatments with rimcazole and its analogs on responding maintained by cocaine injection (0.32 mg/kg per inj) or food presentation (fourth component, each). Ordinates, response rates as percentage of control response rates (sessions before drug tests), which averaged 0.40 (±0.13), and 0.91 (±0.10) response/s, respectively, for cocaine- and food-maintained responding; abscissae, milligrams per kilogram of test compounds administered intraperitoneally, log scale. ●, behavior maintained by cocaine (0.32 mg/kg per inj i.v.); ○, behavior maintained by food presentation.
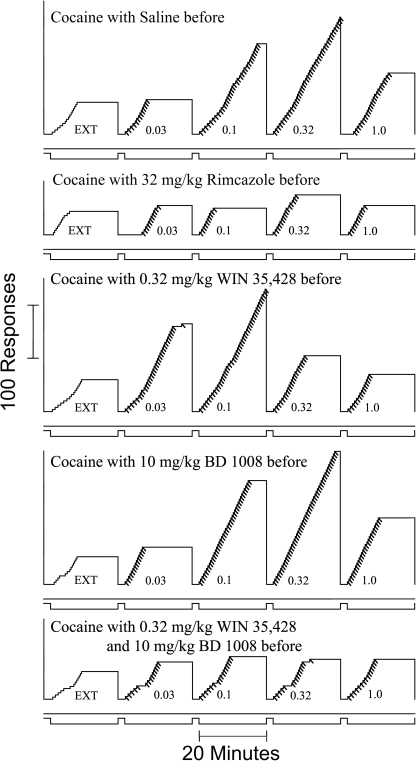
Fig. 4.
Representative cumulative records showing patterns of self-administration maintained by intravenous cocaine injection under the fixed-ratio 5 response schedule with the particular treatments before sessions as indicated in each panel of the figure. All details are as in the legend to Fig. 2D.
The rimcazole analogs, SH 3-24 and SH 3-28, like the parent compound, dose-dependently decreased response rates maintained by cocaine (Fig. 3, B and C, respectively). At intermediate doses of either compound, the maximal effect of cocaine was significantly decreased, and at the highest doses rates of responding were not greater than those obtained in EXT (Fig. 3, B and C, compare ▿ with ● and with levels maintained in EXT). An ANOVA of the effects on response rates of each of these drugs indicated significant effects of cocaine dose (F4,60 = 8.15 and 6.81, respectively, p < 0.001), treatment (F3,60 = 4.58 and 5.76, respectively, p ≤ 0.018), and the interaction of the two (F12,60 = 4.04 and 4.45, respectively, p < 0.001).
The effects of rimcazole on responding maintained at the highest response rates by cocaine (at 0.32 mg/kg per inj, fourth component) were compared with effects on responding maintained by food reinforcement at a comparable point in the session (Fig. 3D). Rimcazole produced dose-dependent decreases in rates of responding maintained by cocaine at doses that had no effect on response rates maintained by food reinforcement (Fig. 3D, compare ● and ○). Two-way repeated-measures ANOVA indicated a significant effect of reinforcer (F1,20 = 30.1, p < 0.001) and a nonsignificant effect of rimcazole dose (F2,20 = 1.53, p = 0.241), although there was a significant interaction of the two (F2,20 = 5.18, p = 0.015). Post hoc analysis indicated that the effects of 32 mg/kg rimcazole on rates of responding maintained by food and cocaine were significantly different (t = 4.60, p < 0.001).
SH 3-24 and SH 3-28 also had selective effects on responding maintained by cocaine (Fig. 3, E and F, respectively). Little, if any, effect on response rates maintained by food reinforcement were obtained (○) across the range of doses that decreased responding maintained by cocaine. For SH 3-24, ANOVA indicated a significant effect of dose (F2,20 = 6.31, p = 0.008), reinforcer (F1,20 = 8.30, p = 0.016), and their interaction (F1,20 = 10.4; p < 0.001). For SH 3-28, the ANOVA indicated a nonsignificant effect of dose (F1,20 = 1.30, p = 0.294) but a significant effect of reinforcer (F1,20 = 21.8, p < 0.001) and a nonsignificant effect of the interaction (F2,20 = 1.58, p = 0.231).
Pretreatment with Selective σR Antagonists or DAT Inhibitors.
The selective effects of rimcazole analogs on cocaine self-administration prompted studies to assess the relative contributions to their effects of actions at σRs and the DAT using compounds with selectivity for those targets. In contrast to the effects of rimcazole and its analogs, the selective σR antagonists, AC927 and NE-100 (Table 1), generally had no significant effects on the self-administration of cocaine (Fig. 5, A and B). Similar results were reported previously with the selective σR antagonists, BD 1008, BD 1047, and BD 1063 (Hiranita et al., 2010). Two-way ANOVA of the effects of AC927 on response rates indicated a significant effect of cocaine dose (F4,60 = 4.66, p = 0.008), with nonsignificant effects of AC927 pretreatment (F3,60 = 0.944, p = 0.444) and the interaction of the two factors (F12,60 = 1.16, p = 0.33). Likewise, the two-way ANOVA of the effect of NE-100 on response rates indicated a significant effect of cocaine dose (F4,60 = 5.23, p = 0.005), but the effects of neither presession treatment (F3,60 = 0.215, p = 0.884) nor the interaction of the two (F12,60 = 0.373, p = 0.968) were significant.
Fig. 5.
Effects of presession treatments with σR antagonists or dopamine uptake inhibitors on cocaine self-administration in rats trained to self-administer cocaine. Ordinates, responses per second; abscissae, cocaine injection dose in milligrams per kilogram. Each point represents the mean ± S.E.M. (n = 6). AC927 was administered intraperitoneally at 15 min before sessions, whereas the other drugs were administered at 5 min before sessions. A, effects of the σR antagonist AC927 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration. B, effects of NE-100 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration. C, effects of WIN 35,428 (0.1, 0.32, and 1.0 mg/kg) on cocaine self-administration. D, effects of methylphenidate (1.0, 3.2, and 10 mg/kg) on cocaine self-administration. E, effects of nomifensine (0.32, 1.0, and 3.2 mg/kg) on cocaine self-administration.
Presession treatment with WIN 35,428 produced a dose-dependent leftward shift in the cocaine self-administration dose-effect curve, without affecting the maximum response rate (Fig. 5C). The lowest dose was behaviorally inactive; 0.32 mg/kg WIN 35,428 shifted the cocaine dose-effect curve approximately 3-fold leftward (Fig. 4, third panel), and 1.0 mg/kg WIN 35,428 shifted the cocaine dose-effect curve approximately 10-fold leftward and produced an increase in response rates during EXT. The ANOVA results of WIN 35,428 pretreatments indicated significant effects of cocaine dose (F4,60 = 4.47, p = 0.010); however, the effect of the presession WIN 35,428 treatment did not achieve statistical significance (F3,60 = 1.22, p = 0.335). However, there was a significant interaction of cocaine dose and WIN 35,428 pretreatment (F12,60 = 2.99, p = 0.002). In addition, post hoc tests indicated that 1.0 mg/kg WIN 35,428 increased response rates maintained by 0.03 mg/kg per inj cocaine (t = 3.76, p = 0.002) and that 0.32 and 1.0 mg/kg WIN 35,428 decreased response rates at a cocaine dose of 0.32 mg/kg per inj (t = 2.75 and 3.19, p = 0.047 and 0.013, respectively).
Presession treatments with methylphenidate or nomifensine also produced leftward shifts in the cocaine self-administration dose-effect curve, without affecting the maximum response rate (Fig. 5, D and E). The lowest doses of both drugs were behaviorally inactive, and doses of 3.2 mg/kg methylphenidate and 1.0 mg/kg nomifensine produced approximate 3-fold leftward shifts in the cocaine dose-effect curve, with higher doses producing further shifts, as well as increasing response rates during EXT. The results of ANOVA indicated significant effects of cocaine dose (F4,60 = 7.52, p < 0.001; F4,60 = 11.4, p < 0.001) and methylphenidate but not nomifensine pretreatment (F3,60 = 4.28, p = 0.023; F3,60 = 0.922, p = 0.454). With both uptake inhibitors, there was a significant interaction of cocaine dose and pretreatment dose (F12,60 = 7.62, p < 0.001; F12,60 = 13.4, p < 0.001). In addition, post hoc tests indicated significant increases in rates of responding maintained with the lowest dose of cocaine by methylphenidate doses of 3.2 and 10 mg/kg (t = 3.41 and 4.93, p ≤ 0.007, respectively).and nomifensine doses of 1.0 and 3.2 mg/kg (t = 4.46 and 6.38, p < 0.001, respectively).
Pretreatment with Combinations of Selective σR Antagonists or DAT Inhibitors.
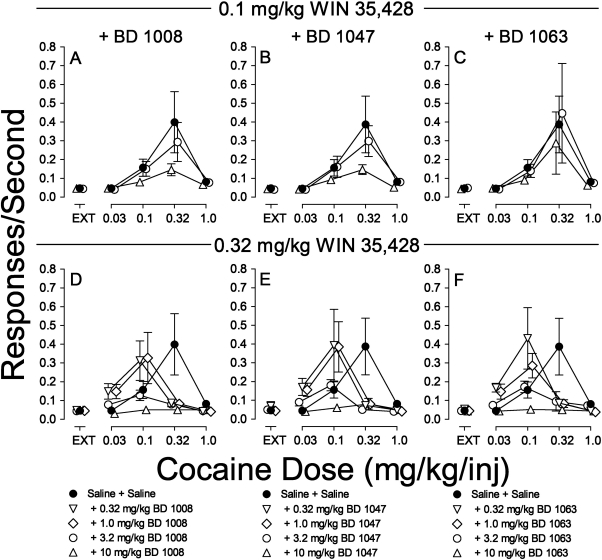
Because neither the selective σR antagonists nor the DAT inhibitors reproduced the effects of rimcazole analogs on responding maintained by cocaine, further studies examined whether combinations of those compounds would reproduce the effects of the rimcazole analogs. The selective σR antagonists used in combination with DAT inhibitors were well studied antagonists synthesized by de Costa et al. (1992, 1993). These compounds are more widely used as selective σR antagonists and were used in our previous study with methods identical to those used here (Hiranita et al., 2010). When administered before sessions in combination with 0.1 mg/kg WIN 35,428, BD 1008 dose-dependently decreased responding maintained by cocaine (Fig. 6A). This effect was obtained despite the fact that the dose of WIN 35,428 as well as all doses of BD 1008 (Hiranita et al., 2010) were behaviorally inactive when administered alone. The ANOVA results (Table 2) indicate significant effects of cocaine dose, presession BD 1008 dose in combination with 0.1 mg/kg WIN 35,428, and their interaction. A similar pattern of significant results was obtained with combinations of 0.1 mg/kg WIN 35,428 and BD 1047 (Fig. 6B; Table 2). In contrast, neither the effects of pretreatment with BD 1063 and 0.1 mg/kg WIN 35,428 nor the interaction of that term with cocaine dose were significant (Fig. 6C; Table 2).
Fig. 6.
Effects of presession treatments with WIN 35,428 combined with σR antagonists on cocaine self-administration. Ordinates, responses per second; abscissae, cocaine injection dose in milligrams per kilogram. Each point represents the mean ± S.E.M. (n = 6). WIN 35,428, BD1008, BD1047, and BD1063 were administered intraperitoneally at 5, 5, 15, and 5 min before sessions, respectively. A, effects of WIN 35,428 (0.1 mg/kg) with BD 1008 (3.2 and 10 mg/kg) on cocaine self-administration. B, effects of WIN 35,428 (0.1 mg/kg) with BD 1047 (3.2 and 10 mg/kg) on cocaine self-administration. C, effects of WIN 35,428 (0.1 mg/kg) with BD 1063 (3.2 and 10 mg/kg) on cocaine self-administration. D, effects of WIN 35,428 (0.32 mg/kg) with BD 1008 (0.32, 1.0, 3.2, and 10 mg/kg) on cocaine self-administration. E, effects of WIN 35,428 (0.32 mg/kg) with BD 1047 (0.32, 1.0, 3.2, and 10 mg/kg) on cocaine self-administration. F, effects of WIN 35,428 (0.32 mg/kg) with BD 1063 (0.32, 1.0, 3.2, and 10 mg/kg) on cocaine self-administration.
TABLE 2.
Results of statistical analysis of the effects of drug combinations on cocaine self-administration
| Treatment | Cocaine Dose | Pretreatment | Interaction | Post Hoc Test |
|
|---|---|---|---|---|---|
| At 0.1 mg/kg per Injection Cocaine | At 0.32 mg/kg per Injection Cocaine | ||||
| 0.1 mg/kg WIN 35,428 and BD 1008 | F4,40 = 6.38, p = 0.002 | F2,40 = 4.51, p = 0.040 | F8,40 = 3.57, p = 0.003 | N.S. | 10 mg/kg BD 1008, t = 5.84, p < 0.001 |
| 0.1 mg/kg WIN 35,428 and BD 1047 | F4,40 = 21.8, p < 0.001 | F2,40 = 1.22, p = 0.337 | F8,40 = 1.94, p = 0.081 | N.S. | N.S. |
| 0.1 mg/kg WIN 35,428 and BD 1063 | F4,40 = 4.15, p = 0.013 | F2,40 = 0.601, p = 0.567 | F8,40 = 0.63, p = 0.748 | N.S. | N.S. |
| 0.3 mg/kg WIN 35,428 and BD 1008 | F4,80 = 6.91, p = 0.001 | F4,80 = 5.68, p = 0.003 | F16,80 = 4.62, p < 0.001 | 1.0 mg/kg BD 1008, t = 3.00, p = 0.034 | 0.32–10 mg/kg BD 1008, t = 5.50, 5.59, 5.62, and 6.12, respectively, p <0.001 |
| 0.3 mg/kg WIN 35,428 and BD 1047 | F4,80 = 11.8, p < 0.001 | F4,80 = 1.65, p = 0.201 | F16,80 = 2.97, p < 0.001 | 0.32 mg/kg BD 1047, t = 4.03, p ≤ 0.002 | N.S. |
| 1.0 mg/kg BD 1047, t = 3.90, p ≤ 0.002 | |||||
| 0.3 mg/kg WIN 35,428 and BD 1063 | F4,80 = 14.2, p < 0.001 | F4,80 = 3.33, p = 0.030 | F16,80 = 4.54, p < 0.001 | 0.32 mg/kg BD 1063, t = 6.09, p ≤ 0.009 | N.S. |
| 1.0 mg/kg BD 1063, t = 3.42, p ≤ 0.009 | |||||
| 1.0 mg/kg MPD and BD 1008 | F4,60 = 8.54, p < 0.001 | F3,60 = 8.06, p = 0.002 | F12,60 = 7.27, p < 0.001 | 3.2 mg/kg BD 1008, t = 3.15, p ≤ 0.016 | 3.2 mg/kg BD 1008, t = 4.54, p < 0.001 |
| 10 mg/kg BD 1008, t = 4.37, p ≤ 0.016 | 10 mg/kg BD 1008, t = 6.62, p < 0.001 | ||||
| 1.0 mg/kg MPD and BD 1047 | F4,60 = 8.41, p < 0.001 | F3,60 = 8.98, p = 0.001 | F12,60 = 7.10, p < 0.001 | 3.2 mg/kg BD 1047, t = 4.44, p < 0.001 | 3.2 mg/kg BD 1047, t = 5.99, p < 0.001 |
| 10 mg/kg BD 1047, t = 4.31, p < 0.001 | 10 mg/kg BD 1047, t = 7.02, p < 0.001 | ||||
| 1.0 mg/kg MPD and BD 1063 | F4,60 = 8.99, p < 0.001 | F3,60 = 8.77, p = 0.001 | F12,60 = 7.56, p < 0.001 | 3.2 mg/kg BD 1063, t = 4.24, p < 0.001 | 3.2 mg/kg BD 1063, t = 5.64, p < 0.001 |
| 10 mg/kg BD 1063, t = 4.37, p < 0.001 | 10 mg/kg BD 1063, t = 6.99, p < 0.001 | ||||
| 0.32 mg/kg nomifensine and BD 1008 | F4,60 = 14.7, p < 0.001 | F3,60 = 13.7, p < 0.001 | F12,60 = 12.8, p < 0.001 | 3.2 mg/kg BD 1008, t = 6.11, p < 0.001 | 3.2 mg/kg BD 1008, t = 7.35, p < 0.001 |
| 10 mg/kg BD 1008, t = 5.91, p < 0.001 | 10 mg/kg BD 1008, t = 9.00, p < 0.001 | ||||
| 0.32 mg/kg nomifensine and BD 1047 | F4,60 = 13.3, p < 0.001 | F3,60 = 12.9, p = 0.001 | F12,60 = 11.8, p < 0.001 | 3.2 mg/kg BD 1047, t = 4.63, p < 0.001 | 3.2 mg/kg BD 1047, t = 5.26, p < 0.001 |
| 10 mg/kg BD 1047, t = 5.77, p < 0.001 | 10 mg/kg BD 1047, t = 8.26, p < 0.001 | ||||
| 0.32 mg/kg nomifensine and BD 1063 | F4,60 = 16.3, p < 0.001 | F3,60 = 14.9, p = 0.001 | F12,60 = 13.3, p < 0.001 | 3.2 mg/kg BD 1063, t = 6.19, p < 0.001 | 3.2 mg/kg BD 1063, t = 7.31, p < 0.001 |
| 10 mg/kg BD 1063, t = 6.30, p < 0.001 | 10 mg/kg BD 1063, t = 9.22, p < 0.001 | ||||
N.S., not significant.
In addition, the effects of a higher dose of WIN 35,428 (0.32 mg/kg) were studied with each of the σR antagonists (Fig. 6, D–F). That dose of WIN 35,428 in combination with low doses of BD 1008 (0.32 and 1.0 mg/kg) shifted the cocaine self-administration dose-effect curve leftward, as did that dose of WIN 35,428 alone. However, higher doses of BD 1008 (3.2 and 10.0 mg/kg) resulted in a decrease in the maximum effect of cocaine dose per injection (Fig. 6D; see also Fig. 4, bottom panel). The ANOVA indicated a significant effect of cocaine dose, presession cotreatment, and their interaction (Table 2). The leftward shift was substantiated by post hoc tests showing significant effects of 0.32 mg/kg WIN 35,428 with the 1.0 mg/kg dose of BD 1008 at the 0.1 mg/kg per inj cocaine dose (Fig. 6D; Table 2). Similar patterns of effects of combinations of the 0.32 mg/kg dose of WIN 35,428 with BD 1047 and BD 1063 were obtained (Fig. 6, E and F), with similar statistical outcomes (Table 2).
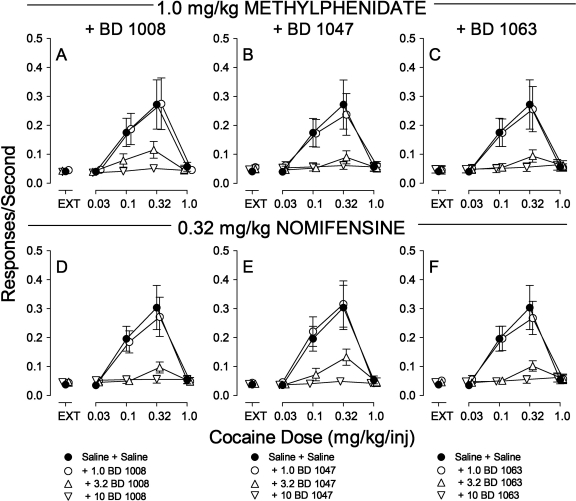
To assess whether the effects of combinations of WIN 35,428 with the various σR antagonists were idiosyncratic to that particular DAT inhibitor, combinations of BD 1008, BD 1047, or BD 1063 with the DAT inhibitors, methylphenidate (Fig. 7, A–C) or nomifensine (Fig. 7, D–F), were studied. The doses of the DAT inhibitors chosen for study were those that did not significantly alter the cocaine self-administration dose-effect curve when administered alone (Fig. 5). As with WIN 35,428, doses of either methylphenidate or nomifensine that were behaviorally inactive when administered alone, allowed previously inactive σR antagonists to produce dose-dependent decreases in the maximal effects of cocaine (Fig. 7). ANOVAs of the effects of 1.0 mg/kg methylphenidate or 0.32 mg/kg nomifensine with BD 1008, BD 1047, or BD 1063 on response rate showed significant effects of cocaine dose, presession treatments, and their interactions (Table 2).
Fig. 7.
Effects of presession treatments with methylphenidate (1.0 mg/kg) or nomifensine (0.32 mg/kg) combined with σR antagonists on cocaine self-administration. Ordinates, responses per second; abscissae, cocaine injection dose in milligrams per kilogram. Each point represents the mean ± S.E.M. (n = 6). Methylphenidate, BD1008, BD1047, and BD1063 were administered intraperitoneally at 5, 5, 15, and 5 min before sessions, respectively. A, effects of methylphenidate (1.0 mg/kg) with BD 1008 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration. B, effects of methylphenidate with BD 1047 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration. C, effects of methylphenidate with BD 1063 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration. D, effects of nomifensine with BD 1008 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration. E, effects of nomifensine with BD 1047 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration. F, effects of nomifensine with BD 1063 (1.0, 3.2, and 10 mg/kg) on cocaine self-administration.
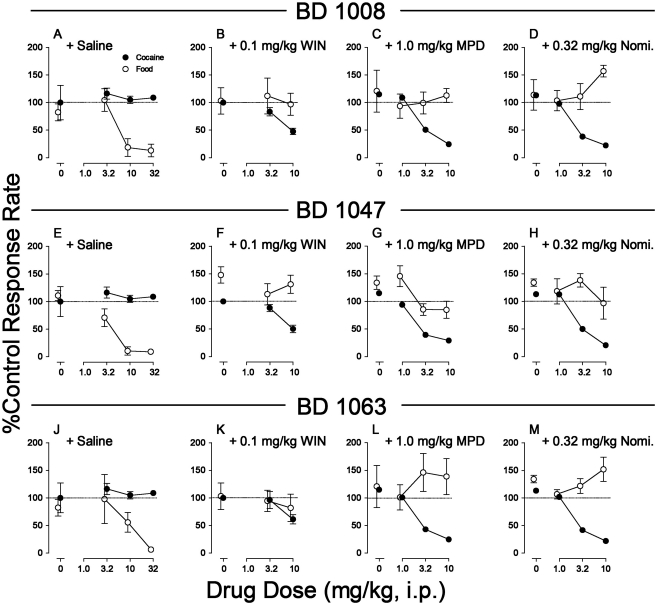
The effects of combined pretreatment with dopamine uptake inhibitors and σR antagonists (BD 1008, BD 1047, and BD 1063) on responding maintained at the highest response rates by cocaine (at 0.32 mg/kg per inj, fourth component) were compared with effects on responding maintained by food reinforcement at a comparable point in the session (Fig. 8). As shown in our previous study (Hiranita et al., 2010), the σR antagonists alone had no effect on responding maintained by cocaine at doses that dose-dependently decreased rates of responding maintained by food reinforcement (Fig. 8, left column; results of statistical analyses can be found in Table 3). In contrast, combined treatment with a DAT inhibitor and a σR antagonist reversed the selectivity of the effects obtained with the σR antagonist alone; rates of responding maintained by cocaine were decreased at doses of the σR antagonist combined with the DAT inhibitor that were ineffective on rates of responding maintained by food (Fig. 8, B–D). Informal observations indicated that subjects responding under the schedule of food presentation consumed the pellets delivered during sessions.
Fig. 8.
Effects of presession treatments with dopamine uptake inhibitors combined with σR antagonists on responding maintained by cocaine injection or food presentation. Ordinates, response rates as percentage of control response rates (sessions before drug tests); abscissae, milligrams per kilogram σR antagonists administered intraperitoneally in combination with designated dopamine uptake inhibitors, log scale. A, effects of BD 1008 with saline. B, effects of BD 1008 with WIN 35,428 (0.1 mg/kg). C, effects of BD 1008 with methylphenidate (1.0 mg/kg). D, effects of BD 1008 with nomifensine (0.32 mg/kg). E, effects of BD 1047 with saline. F, effects of BD 1047 with WIN 35,428 (0.1 mg/kg). G, effects of BD 1047 with methylphenidate (1.0 mg/kg). H, effects of BD 1047 with nomifensine (0.32 mg/kg). J, effects of BD 1063 with saline. K, effects of BD 1063 with WIN 35,428 (0.1 mg/kg). L, effects of BD 1063 with methylphenidate (1.0 mg/kg). M, effects of BD 1063 with nomifensine (0.32 mg/kg). Dopamine uptake inhibitors and σR antagonists were administered intraperitoneally 5 min before sessions except BD 1047 (15 min). Responding was from the fourth 20-min component of the session (see Materials and Methods). All other details are as in the legend to Fig. 3, D–F.
TABLE 3.
Results of statistical analysis of the effects of drug combinations on cocaine self-administration compared with food-maintained behavior
| Treatment | Reinforcer | Dose | Interaction | Post Hoc Test of Reinforcer |
|---|---|---|---|---|
| BD 1008 and saline | F1,15 = 21.5, p < 0.001 | F2,15 = 5.88, p = 0.013 | F2,15 = 0.675, p = 0.524 | 10 mg/kg BD 1008, t = 2.67, p = 0.012 |
| 32 mg/kg BD 1008, t = 3.04, p = 0.005 | ||||
| BD 1047 and saline | F1,15 = 99.1, p < 0.001 | F2,15 = 0.485, p = 0.625 | F2,15 = 4.68, p = 0.026 | 10 mg/kg BD 1047, t = 5.78, p < 0.001 |
| 32 mg/kg BD 1047, t = 6.38, p < 0.001 | ||||
| BD 1063 and saline | F1,20 = 5.01, p = 0.049 | F2,20 = 4.72, p = 0.021 | F2,20 = 2.83, p = 0.083 | 32 mg/kg BD 1063, t = 2.98, p = 0.006 |
| 0.1 mg/kg WIN 35,428 and BD 1008 | F1,10 = 2.57, p = 0.140 | F1,10 = 3.65, p = 0.085 | F1,10 = 0.598, p = 0.457 | N.S. |
| 0.3 mg/kg WIN 35,428 and BD 1008 | F1,30 = 16.2, p = 0.002 | F3,30 = 0.944, p = 0.432 | F3,30 = 0.640, p = 0.595 | 0.32 mg/kg BD 1008, t = 2.52, p = 0.018 |
| 1.0 mg/kg BD 1008, t = 2.89, p = 0.008 | ||||
| 3.2 mg/kg BD 1008, t = 3.91, p < 0.001 | ||||
| 10 mg/kg BD 1008, t = 2.75, p = 0.011 | ||||
| 1.0 mg/kg methylphenidate and BD 1008 | F1,20 = 10.9, p = 0.008 | F2,20 = 4.01, p = 0.034 | F2,20 = 9.19, p = 0.001 | 3.2 mg/kg BD 1008, t = 2.60, p = 0.014 |
| 10 mg/kg BD 1008, t = 4.73, p < 0.001 | ||||
| 0.32 mg/kg nomifensine and BD 1008 | F1,20 = 24.9, p < 0.001 | F2,20 = 3.25, p = 0.060 | F2,20 = 19.7, p < 0.001 | 3.2 mg/kg BD 1008, t = 3.92, p < 0.001 |
| 10 mg/kg BD 1008, t = 7.27, p < 0.001 | ||||
| 0.1 mg/kg WIN 35,428 and BD 1047 | F1,10 = 7.95, p = 0.018 | F1,10 = 7.55, p = 0.021 | F1,10 = 60.0, p < 0.001 | N.S. |
| 0.3 mg/kg WIN 35,428 and BD 1047 | F1,30 = 158, p < 0.001 | F3,30 = 3.16, p = 0.039 | F3,30 = 0.717, p = 0.550 | 0.32 mg/kg BD 1047, t = 6.02, p < 0.001 |
| 1.0 mg/kg BD 1047, t = 5.83, p < 0.001 | ||||
| 3.2 mg/kg BD 1047, t = 4.20, p < 0.001 | ||||
| 10 mg/kg BD 1047, t = 5.98, p < 0.001 | ||||
| 1.0 mg/kg methylphenidate and BD 1047 | F1,20 = 17.2, p = 0.002 | F2,20 = 34.3, p < 0.001 | F2,20 = 0.166, p = 0.848 | 1.0 mg/kg BD 1047, t = 3.30, p = 0.003 |
| 3.2 mg/kg BD 1047, t = 2.94, p = 0.008 | ||||
| 10 mg/kg BD 1047, t = 3.55, p = 0.002 | ||||
| 0.32 mg/kg nomifensine and BD 1047 | F1,20 = 9.25, p = 0.012 | F2,20 = 14.02, p < 0.001 | F2,20 = 8.50, p = 0.002 | 3.2 mg/kg BD 1047, t = 3.93, p < 0.001 |
| 10 mg/kg BD 1047, t = 3.39, p = 0.003 | ||||
| 0.1 mg/kg WIN 35,428 and BD 1063 | F1,10 = 0.284, p = 0.606 | F1,10 = 1.71, p = 0.220 | F1,10 = 0.375, p = 0.554 | N.S. |
| 0.3 mg/kg WIN 35,428 and BD 1063 | F1,30 = 85.9, p < 0.001 | F3,30 = 0.690, p = 0.565 | F3,30 = 1.27, p = 0.303 | 0.32 mg/kg BD 1063, t = 2.42, p = 0.021 |
| 1.0 mg/kg BD 1063, t = 3.97, p < 0.001 | ||||
| 3.2 mg/kg BD 1063, t = 2.68, p = 0.011 | ||||
| 10 mg/kg BD 1063, t = 5.00, p < 0.001 | ||||
| 1.0 mg/kg methylphenidate and BD 1063 | F1,20 = 19.5, p = 0.001 | F2,20 = 0.377, p = 0.690 | F2,20 = 3.91, p = 0.037 | 3.2 mg/kg BD 1063, t = 3.37, p = 0.002 |
| 10 mg/kg BD 1063, t = 3.72, p < 0.001 | ||||
| 0.32 mg/kg nomifensine and BD 1063 | F1,20 = 70.9, p < 0.001 | F2,20 = 1.95, p = 0.168 | F2,20 = 13.7, p < 0.001 | 3.2 mg/kg BD 1063, t = 4.92, p < 0.001 |
| 10 mg/kg BD 1063, t = 7.99, p < 0.001 |
N.S., not significant.
Combined treatment with BD 1047 (Fig. 8, F–H) or BD 1063 (Fig. 8, K–M) with any of the DAT inhibitors also selectively altered responding maintained by cocaine compared with that maintained by food reinforcement. As with BD 1008, the selective effects of BD 1047 (Fig. 8E) or BD 1063 (Fig. 8J) on responding maintained by food reinforcement were reversed by combined treatment with a DAT inhibitor, and the statistical analyses of the effects of each drug combination indicated significant effects of reinforcer in all cases except with WIN 35,428 and BD 1063 (Table 3). In general, the DAT inhibitors attenuated the effects of the σR antagonists on food-reinforced behavior and increased their effects on responding maintained by cocaine.
Discussion
In the present study, the σR antagonist, rimcazole, and two of its N-propylphenyl analogs dose-dependently decreased rates of cocaine self-administration. These decreases were obtained at doses that did not affect comparable food-reinforced responding. In contrast, although consistent with previous findings (Martin-Fardon et al., 2007; Hiranita et al., 2010), a number of other σR antagonists did not appreciably alter self-administration of cocaine. In the study by Hiranita et al. (2010), presession treatments with selective σR antagonists were without effects on cocaine self-administration up to doses 3-fold higher than those that reliably antagonized the in vivo effects of σR agonists. Likewise, the cocaine-induced increases in extracellular dopamine levels in the nucleus accumbens shell of rats were not altered by the σR antagonists BD 1008 and BD 1063 (Garcés-Ramírez et al., 2011). Because rimcazole analogs differ from σR antagonists in their additional affinity for the DAT, the present study further assessed the relative contributions of these two sites to the blockade of cocaine self-administration.
As was shown previously (Barrett et al., 2004; Hiranita et al., 2009, 2010), DAT inhibitors (WIN 35,428, methylphenidate, and nomifensine) were themselves self-administered, and each shifted the cocaine self-administration dose-effect curve leftward. The decreases in cocaine self-administration with rimcazole analogs show that despite affinity for the DAT, their effects are substantially different from those of the DAT inhibitors. A previous study has also reported the reduced cocaine-like effects of rimcazole analogs (Katz et al., 2003). Acute administration of rimcazole analogs neither stimulated locomotor activity in mice nor produced cocaine-like discriminative stimulus effects in rats. Furthermore, pretreatments with rimcazole analogs blocked cocaine-stimulated locomotor activity in mice (Katz et al., 2003). Thus, rimcazole analogs, although having DAT affinity, generally produce a spectrum of in vivo effects that differ from those of typical DAT inhibitors, such as those presently studied.
Combinations of WIN 35,428 and the σR antagonists BD 1008 and BD 1047 shifted the cocaine self-administration dose-effect curve downward, suggesting a decrease in the reinforcing effects of cocaine. These effects were similar to those of rimcazole analogs and not specific to WIN 35,428, because similar effects were obtained with methylphenidate and nomifensine. Furthermore and as with the effects of rimcazole analogs, the drug combinations selectively decreased cocaine-maintained compared with food-maintained responding. Of interest, it appears that DAT inhibition is also necessary to avoid the effects of σR antagonists on food-maintained responding. Thus, the present results suggest that the effects of rimcazole analogs on cocaine self-administration result from dual occupancy of the DAT and σRs and that these effects may be selective, suggesting further support for developing these agents as treatments for cocaine abuse (Cao et al., 2003; Katz et al., 2003).
The optimal ratio of σR to DAT affinity cannot be completely addressed without resolution of the relative contributions to the observed effects of σ1 and σ2 receptors (see below). From Table 1 it can be seen that the compounds with cocaine-antagonist effects had in vitro σ1R/DAT affinity ratios between 2 and 10 and in vitro σ2R/DAT affinity ratios between 2 and 4. As with the selective DAT inhibitors, drugs with high σ1R/DAT affinity ratios would be expected to produce leftward shifts, suggesting a potentiation of the reinforcing effects of cocaine. However, if the ratio is not excessively weighted toward DAT, the leftward shift would be expressed along with downward shifts, as was obtained with the higher dose of WIN 35,428 in combination with adequate doses of the σR antagonists. The relative dominance of leftward or downward shifts would be expected to vary with the σR/DAT affinity ratio.
It may be noteworthy that higher doses of BD 1008, BD 1047, and possibly BD 1063 were necessary to antagonize cocaine self-administration in combination with WIN 35,428 than with the other DAT inhibitors. Furthermore, the σ2R affinity of WIN 35,428 is roughly 10-fold higher than that of the other DAT inhibitors, although their σ1R affinities are similar. It is possible that WIN 35,428 has σR agonist effects, like cocaine (Su and Hayashi, 2001), and that these effects must be overcome by the σR antagonists to produce effects like those of the rimcazole analogs. The lower affinity of nomifensine and methylphenidate at σ2Rs compared with that of WIN 35,428 may result in the lower necessary combination doses of the σR antagonists. Furthermore, the σR antagonist with preferential σ1 affinity (BD 1063) was least effective in selectively decreasing cocaine self-administration in combination with WIN 35,428 than were the other σR antagonists. Differences between BD 1063 and the other σR antagonists were not evident with nomifensine and methylphenidate, DAT inhibitors that had low affinities at both σ1 and σ2 receptors. Thus, antagonist actions at σ2Rs may be more involved in the interaction than the σ1 subtype. Nevertheless, a clear resolution of the relative contributions of the subtypes of σRs awaits more selective antagonists (Mésangeau et al., 2008).
Previous studies have indicated that cocaine-like dopamine uptake inhibitors bind to the DAT in an outward-facing conformation (Ferrer and Javitch, 1998; Loland et al., 2002), whereas rimcazole analogs bind preferentially to an inward-facing conformation (Loland et al., 2008). Decreases in potency for the inhibition of dopamine uptake in cells transfected with wild-type DAT compared with a Y335A point-mutated DAT, which assumes an inward-facing conformation, predict whether the compounds will be cocaine-like or atypical in their behavioral effects. Those exhibiting larger decreases in potency are cocaine-like, whereas those that show small changes have reduced cocaine-like effects. WIN 35,428 was among the compounds showing large changes in potency, whereas rimcazole analogs showed much smaller changes in potency (Loland et al., 2008). Thus, DAT conformational equilibrium shifts favored by distinct DAT inhibitors may be related to whether those compounds will produce typical cocaine-like effects. However, WIN 35,428 typically produces effects like those of cocaine and shows a marked decrease in potency in the DAT Y335A mutant compared with the wild type (Loland et al., 2008). In addition, both nomifensine and methylphenidate produce effects characteristic of cocaine-like DAT inhibitors, indicating that typical DAT inhibitors can produce effects like those of the atypical DAT inhibitors when administered in combination with σR antagonists. Thus, DAT inhibitors inducing an outward-facing DAT conformational equilibrium may produce atypical effects like those of rimcazole when the σR is antagonized.
Previous studies have indicated that endogenous zinc can shift equilibrium of the DAT to an outward-facing conformation (Loland et al., 2002). Furthermore, zinc has been implicated as an endogenous σ2R ligand (Connor and Chavkin, 1992). Thus, any action that mobilizes endogenous zinc (Frederickson et al., 2005) could alter both the DAT and σRs, although how those actions combine to regulate the effects of cocaine is currently speculative.
Another potential contribution to the interaction between σRs and the DAT is membrane cholesterol. Several studies have documented an involvement of both σ1 (Hayashi and Su, 2005) and σ2 (Gebreselassie and Bowen, 2004) receptors in intracellular lipid dynamics. Stimulation of the σ1R facilitates its translocation to the plasmalemma membrane within which lipid rafts may alter the function of membrane-bound proteins (Hayashi and Su, 2003). Previous studies have demonstrated an influence of membrane lipids on DAT trafficking (Foster et al., 2008) and transport capacity (Adkins et al., 2007). More recently, Hong and Amara (2010) have suggested on the basis of substituted cysteine accessibility methods that a cholesterol-rich membrane environment shifts the DAT conformational equilibrium toward a state accessible to the extracellular space (outward-facing), as does zinc. Again, the potential of an interaction between σRs and the DAT involving membrane cholesterol is currently speculative.
Indirect dopamine agonist actions mediated by the dopamine D1R have also been recently shown to be influenced by actions at σRs. A recent report by Fu et al. (2010) indicates that the σR agonist, PRE-084, which had no effects of its own, amplified the effects of the D1R partial agonist, SKF 38393. In addition, Navarro et al. (2010) showed evidence supporting heteromerization of σ and dopamine D1 receptors and a potentiation of D1R-mediated adenylyl cyclase activation by actions at σRs. The reported interactions among σ and dopamine D1 receptors were also characterized by mutual antagonism of mitogen-activated protein kinase activation by cocaine acting at σRs and the D1 agonist, SKF 81297. These studies, including those on zinc and lipids, together suggest several potential mechanisms through which σRs may, along with DAT inhibitors, modulate the reinforcing effects of cocaine.
Thus, the present study identifies compounds acting on both the DAT and σRs to decrease the self-administration of cocaine. These compounds were not self-administered themselves, suggesting low abuse liability of their own. Clinical trials with rimcazole as a potential antipsychotic agent indicated a lack of efficacy (Borison et al., 1991) and an incidence of seizures that halted its further development. Although rimcazole, therefore, may not be an ideal candidate for development as a treatment for cocaine dependence, the present studies suggest that dual inhibition of DAT and σRs can more than adequately blunt the abuse liability resulting from DAT inhibition alone and further suggest that actions at both sites substantially and selectively block the reinforcing effect of cocaine.
Acknowledgments
We thank Patty Ballerstadt for administrative assistance and Drs. Tsung-Ping Su and Andrew Coop for generous gifts of compounds. Dr. Su also provided valuable advice during the conduct of these studies.
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse.
Parts of this study were presented previously: Hiranita T, Tanda G, Kopajtic T, Newman AH, and Katz JL (2009) Reinforcing effects of sigma-receptor agonists in cocaine-experienced and naïve rats. Neuroscience 2009; 2009 Oct 17–21; Chicago, IL. Society for Neuroscience, Washington, DC; and Hiranita T, Tanda G, Kopajtic T, Newman AH, and Katz JL; Decreases in cocaine self-administration by dual inhibition of the dopamine transporter and antagonism of sigma receptors. Behavioral Pharmacology Society 2010; 2010 Apr 23–24; Anaheim, CA. Behavioral Pharmacology Society.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.185025.
- σR
- σ receptor
- BMY 14802
- α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazine-butanol
- BD 1047
- N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino) ethylamine dihydrobromide
- NE-100
- N,N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]-ethylamine monohydrochloride
- BD 1008
- N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine dihydrobromide
- BD 1063
- N-[2-(3,4-dichlorophenyl) ethyl]-4-methylpiperazine dihydrochloride
- DAT
- dopamine transporter
- SH 3-24
- [3-(cis-3,5-dimethyl-4-[3-phenylpropyl]-1-piperazinyl)-propyl]diphenylamine hydrochloride
- SH 3-28
- 9-[3-(cis-3,5-dimethyl-4-[3-phenylpropyl]-1-piperazinyl)-propyl]carbazole hydrobromide
- LED
- light-emitting diode
- TO
- timeout
- EXT
- extinction
- inj
- injection
- WIN 35,428
- 2β-carbomethoxy-3β-(4-fluorophenyl)tropane
- AC927
- N-phenethylpiperidine oxalate
- OWW
- original wet weight
- DTG
- 1,3-di-(2-tolyl)guanidine
- NIDA
- National Institute on Drug Abuse
- ANOVA
- analysis of variance
- D1R
- dopamine D1 receptor
- PRE-084
- 2-(4-morpholinethyl) 1-phenylcyclohexane-1-carboxylate hydrochloride
- SKF 38393
- 2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepine
- SKF 81297
- R(+)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide.
Authorship Contributions
Participated in research design: Hiranita and Katz.
Conducted experiments: Hiranita and Kopajtic.
Contributed new reagents or analytic tools: Cao and Newman.
Performed data analysis: Hiranita and Katz.
Wrote or contributed to the writing of the manuscript: Hiranita, Soto, Kohut, Kopajtic, Cao, Newman, Tanda, and Katz.
References
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, Ramamoorthy S, Gether U. (2007) Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry 46:10484–10497 [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. (2004) Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology 47 (Suppl 1):256–273 [DOI] [PubMed] [Google Scholar]
- Borison RL, Diamond BI, Dren AT. (1991) Does σ receptor antagonism predict clinical antipsychotic efficacy? Psychopharmacol Bull 27:103–106 [PubMed] [Google Scholar]
- Cao J, Kulkarni SS, Husbands SM, Bowen WD, Williams W, Kopajtic T, Katz JL, George C, Newman AH. (2003) Dual probes for the dopamine transporter and σ1 receptors: novel piperazinyl alkyl-bis(4′-fluorophenyl)amine analogues as potential cocaine-abuse therapeutic agents. J Med Chem 46:2589–2598 [DOI] [PubMed] [Google Scholar]
- Connor MA, Chavkin C. (1992) Ionic zinc may function as an endogenous ligand for the haloperidol-sensitive σ2 receptor in rat brain. Mol Pharmacol 42:471–479 [PubMed] [Google Scholar]
- de Costa BR, He XS, Linders JT, Dominguez C, Gu ZQ, Williams W, Bowen WD. (1993) Synthesis and evaluation of conformationally restricted N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamines at σ receptors. 2. Piperazines, bicyclic amines, bridged bicyclic amines, and miscellaneous compounds. J Med Chem 36:2311–2320 [DOI] [PubMed] [Google Scholar]
- de Costa BR, Radesca L, Di Paolo L, Bowen WD. (1992) Synthesis, characterization, and biological evaluation of a novel class of N-(arylethyl)-N-alkyl-2-(1-pyrrolidinyl)ethylamines: structural requirements and binding affinity at the σ receptor. J Med Chem 35:38–47 [DOI] [PubMed] [Google Scholar]
- Ferrer JV, Javitch JA. (1998) Cocaine alters the accessibility of endogenous cysteines in putative extracellular and intracellular loops of the human dopamine transporter. Proc Natl Acad Sci USA 95:9238–9243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. (2008) Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem 105:1683–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462 [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhao Y, Luan W, Dong LY, Dong Y, Lai B, Zhu Y, Zheng P. (2010) Sigma-1 receptors amplify dopamine D1 receptor signaling at presynaptic sites in the prelimbic cortex. Biochim Biophys Acta 1803:1396–1408 [DOI] [PubMed] [Google Scholar]
- Garcés-Ramírez L, Green JL, Hiranita T, Kopajtic TA, Mereu M, Thomas AM, Mesangeau C, Narayanan S, McCurdy CR, Katz JL, et al. (2011) Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis in rats. Biol Psychiatry 69:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreselassie D, Bowen WD. (2004) Sigma-2 receptors are specifically localized to lipid rafts in rat liver membranes. Eur J Pharmacol 493:19–28 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alvear GM, Werling LL. (1994) Regulation of [3H]dopamine release from rat striatal slices by sigma receptor ligands. J Pharmacol Exp Ther 271:212–219 [PubMed] [Google Scholar]
- Hayashi T, Maurice T, Su TP. (2000) Ca2+ signaling via σ1-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration. J Pharmacol Exp Ther 293:788–798 [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2003) Sigma-1 receptors (σ1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther 306:718–725 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2005) The potential role of sigma-1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci 77:1612–1624 [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. (2009) Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. (2010) Reinforcing effects of σ-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther 332:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong WC, Amara SG. (2010) Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J Biol Chem 285:32616–32626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husbands SM, Izenwasser S, Kopajtic T, Bowen WD, Vilner BJ, Katz JL, Newman AH. (1999) Structure-activity relationships at the monoamine transporters and sigma receptors for a novel series of 9-[3-(cis-3,5-dimethyl-1-piperazinyl)propyl]carbazole (rimcazole) analogues. J Med Chem 42:4446–4455 [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Newman AH, Katz JL. (1993) Cocaine and several sigma receptor ligands inhibit dopamine uptake in rat caudate-putamen. Eur J Pharmacol 243:201–205 [DOI] [PubMed] [Google Scholar]
- Katz JL, Libby TA, Kopajtic T, Husbands SM, Newman AH. (2003) Behavioral effects of rimcazole analogues alone and in combination with cocaine. Eur J Pharmacol 468:109–119 [DOI] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, Kopajtic T, Tsai SY. (2011) A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals 4:880–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. (2008) Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73:813–823 [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Litman T, Gether U. (2002) Generation of an activating Zn2+ switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc Natl Acad Sci USA 99:1683–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. (2007) Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology 32:1967–1973 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. (2009) Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert Rev Clin Pharmacol 2:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. (2001) Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting sigma1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol 419:163–174 [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. (2009) The pharmacology of sigma-1 receptors. Pharmacol Ther 124:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, Katz JL, Witkin JM. (1991) Selective σ ligands block stimulant effects of cocaine. Eur J Pharmacol 201:251–252 [DOI] [PubMed] [Google Scholar]
- Mésangeau C, Narayanan S, Green AM, Shaikh J, Kaushal N, Viard E, Xu YT, Fishback JA, Poupaert JH, Matsumoto RR, et al. (2008) Conversion of a highly selective sigma-1 receptor-ligand to sigma-2 receptor preferring ligands with anticocaine activity. J Med Chem 51:1482–1486 [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J, Cortés A, Casadó V, Canela EI, Ortiz J, et al. (2010) Direct involvement of σ-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci USA 107:18676–18681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME, Berfield JL, Wang LC, Ferrer JV, Javitch JA. (2001) The uptake inhibitors cocaine and benztropine differentially alter the conformation of the human dopamine transporter. J Biol Chem 276:29012–29018 [DOI] [PubMed] [Google Scholar]
- Romieu P, Meunier J, Garcia D, Zozime N, Martin-Fardon R, Bowen WD, Maurice T. (2004) The sigma 1 (σ1) receptor activation is a key step for the reactivation of cocaine conditioned place preference by drug priming. Psychopharmacology (Berl) 175:154–162 [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. (2002) Involvement of the sigma1 receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology 26:444–455 [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T. (2001) Cocaine affects the dynamics of cytoskeletal proteins via sigma1 receptors. Trends Pharmacol Sci 22:456–458 [DOI] [PubMed] [Google Scholar]
- Tam SW. (1983) Naloxone-inaccessible a receptor in rat central nervous system. Proc Natl Acad Sci USA 80:6703–6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Kanzaki A, Okumura K, Akiyama K, Otsuki S. (1992) Sigma (σ) antagonist BMY 14802 prevents methamphetamine-induced sensitization. Life Sci 50:PL129–PL134 [DOI] [PubMed] [Google Scholar]
- Wachtel SR, White FJ. (1988) Electrophysiological effects of BMY 14802, a new potential antipsychotic drug, on midbrain dopamine neurons in the rat: acute and chronic studies. J Pharmacol Exp Ther 244:410–416 [PubMed] [Google Scholar]
- Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, Katz JL. (1993) Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther 266:473–482 [PubMed] [Google Scholar]