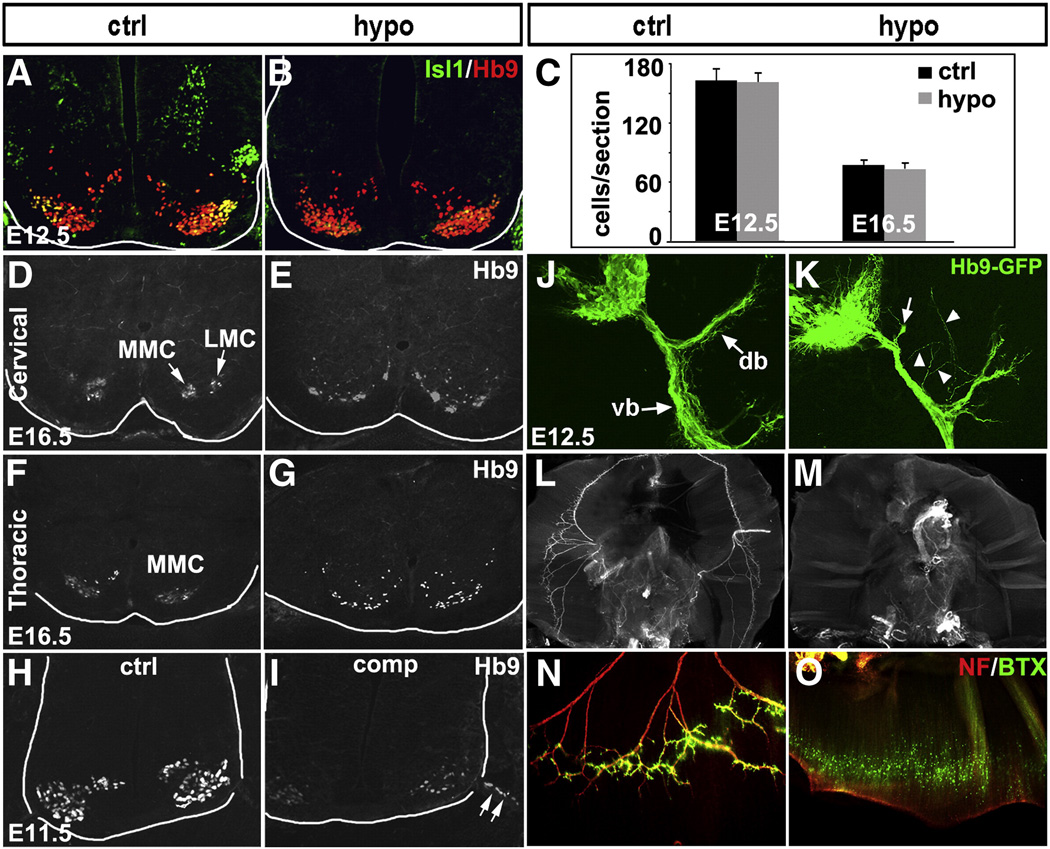
Fig. 5.
Defects in MN migration, motor column formation and axon growth in Isl1 hypomorphic embryos. Despite significant reduction in Isl1 expression in spinal MNs of Isl1 hypomorphic mutant, the level of Hb9 expression and the number of Hb9 expressing MNs during development are not changed (A, B and C). At E16.5, Hb9+ MNs are segregated into medial (MMC) and lateral (LMC) motor columns at the cervical spinal cord, or MMC at the thoracic spinal cord of control mice (D, F). However, in Isl1 hypomorphic embryos, Hb9+ MNs are scattered and ectopically positioned or abnormally clustered and fail to form distinct motor columns (E, G). In Isl1 compound mutant spinal cord, MNs were frequently observed to migrate along ventral roots out of neural tube (H, I arrow). In Hb9-GFP transgenic mice, GFP expression marks the axons of the spinal MNs, which after exit from vertebral column, are divided into dorsal branch (db) and ventral branch (vb) (J). In Isl1 hypomorphic mice that have been crossed onto Hb9-GFP transgenic background, the dorsal branch of the spinal motor axons appeared to be blunt (K, arrow) and frequent ectopic motor projections were observed (K, arrowhead). Wholemount neurofilament immunostaining and Alexa 488-α-bungarotoxin (BTX) staining revealed motor innervation of diaphragm by phrenic nerve and the formation of neuromuscular junctions on the diaphragm of control mice (L, N). However, diaphragm of Isl1 hypomorphic mutant mice were deinnervated and no functional neuromuscular junctions were detected (M, O).