Abstract
The title compound, [Ag(C72H84N4O4)], crystallizes with the AgII cation on a centre of symmetry. The macrocyclic 24-membered ring core is planar with a mean deviation of 0.0311 (15) Å and the four-coordinate AgII cation fits into its center, at 2.0814 (19) and 2.0872 (19) Å, from the surrounding pyrrole-N atoms, in agreement with what is found in related compounds. The p-heptyloxyphenyl groups are rotated 75.51 (5) and 84.45 (8)° with respect to the porphyrin mean plane, due to steric hindrance with the pyrrole-H atoms of the macrocycle.
Related literature
For background information on metalloporphyrins and their derivatives, see: Fu et al. (2009 ▶); Jurow et al. (2010 ▶); Taniguchi & Lindsey (2010 ▶); Zenkevich et al. (2001 ▶). For related structures, see: Scheidt et al. (1986 ▶); Xu et al. (2007 ▶).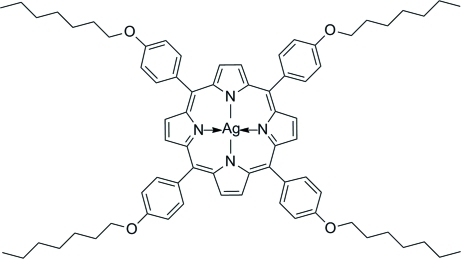
Experimental
Crystal data
[Ag(C72H84N4O4)]
M r = 1177.30
Monoclinic,

a = 15.850 (1) Å
b = 19.1896 (12) Å
c = 10.3285 (7) Å
β = 91.724 (1)°
V = 3140.0 (4) Å3
Z = 2
Mo Kα radiation
μ = 0.37 mm−1
T = 185 K
0.24 × 0.17 × 0.10 mm
Data collection
Bruker APEX CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2004 ▶) T min = 0.916, T max = 0.964
18310 measured reflections
5544 independent reflections
4385 reflections with I > 2σ(I)
R int = 0.035
Refinement
R[F 2 > 2σ(F 2)] = 0.036
wR(F 2) = 0.091
S = 1.02
5544 reflections
369 parameters
H-atom parameters constrained
Δρmax = 0.53 e Å−3
Δρmin = −0.20 e Å−3
Data collection: SMART (Bruker, 2002 ▶); cell refinement: SAINT (Bruker, 2002 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶) and PLATON (Spek, 2009 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681103385X/bg2416sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681103385X/bg2416Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This work was supported by the National Analytical Research Center of Electrochemistry and Spectroscopy, Changchun Institute of Applied Chemistry, Changchun, China.
supplementary crystallographic information
Comment
Porphyrins, metalloporphyrins, and their derivatives are applied in many fields, such as biomimetic catalysts (Fu et al., 2009), molecular electronic components (Jurow et al., 2010), artificial photosynthesis (Taniguchi et al., 2010) or electron transfer and energy migration (Zenkevich et al., 2001). In this paper, the structure of Silver(II)meso-tetrakis[p-(heptyloxy)phenyl]porphyrinate (I) is reported.
The compound crystallizes with the AgII cation in a centre of symmetry (Fig. 1). The macrocyclic 24-membered ring core is planar with a mean deviation of 0.0311 (15) Å and the four coordinate AgII ion fits into its center, at 2.0814 (19) and 2.0872 (19) Å, from the surrouding pyrrole N atoms, in agreement with what found in related compounds (Scheidt et al., 1986; Xu et al., 2007).
The p-heptyloxyphenyl groups are rotated at angles of 75.51 (5)° and 84.45 (8)° with respect to the porphyrin mean plane, due to steric hindrance with the pyrrole-H atoms of the macrocycle.
Experimental
0.03mmol meso-tetrakis[p-(heptyloxy)phenyl] porphyrin and 0.06mmol AgNO3 were dissolved in 20 ml chloroform, refluxed for 6 hours, and the solvent was removed by a rotary evaporator, the residue was purified by column chromatography with chloroform, then recrystallized from a methanol/chloroform solution, and a purple solid was obtained (yield=23%). Single crystals were obtained from recrystallization from a dichloromethane solution at room temperature.
Refinement
H atoms were placed in calculated positions (C—H = 0.95, 0.98 or 0.99 Å) and refined in riding mode, with Uiso(H) = xUeq(C), where x = 1.5 for methyl and 1.2 for all other H atoms.
A Platon run (Spek, 2009) detects solvent accessible voids of 78 Å3, which indicate that the structure may contain disordered solvent molecules (dichloromethane). However, efforts to locate the solvent molecules failed because the residual electron density is small (the highest peak of residual density is 0.529 e Å-3).
Figures
Fig. 1.
A view of (I), with the atom-labeling scheme and 50% probability displacement ellipsoids. Symmetry codes: (i) -x, -y, -z+2.
Crystal data
| [Ag(C72H84N4O4)] | F(000) = 1246 |
| Mr = 1177.30 | Dx = 1.245 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 5112 reflections |
| a = 15.850 (1) Å | θ = 2.2–24.8° |
| b = 19.1896 (12) Å | µ = 0.37 mm−1 |
| c = 10.3285 (7) Å | T = 185 K |
| β = 91.724 (1)° | Block, purple |
| V = 3140.0 (4) Å3 | 0.24 × 0.17 × 0.10 mm |
| Z = 2 |
Data collection
| Bruker APEX CCD diffractometer | 5544 independent reflections |
| Radiation source: fine-focus sealed tube | 4385 reflections with I > 2σ(I) |
| graphite | Rint = 0.035 |
| φ and ω scans | θmax = 25.0°, θmin = 1.7° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2004) | h = −17→18 |
| Tmin = 0.916, Tmax = 0.964 | k = −22→19 |
| 18310 measured reflections | l = −12→12 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.036 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.091 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0483P)2 + 0.7718P] where P = (Fo2 + 2Fc2)/3 |
| 5544 reflections | (Δ/σ)max < 0.001 |
| 369 parameters | Δρmax = 0.53 e Å−3 |
| 0 restraints | Δρmin = −0.20 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ag1 | 0.0000 | 0.0000 | 1.0000 | 0.02274 (9) | |
| C1 | −0.17114 (15) | 0.03711 (13) | 1.1183 (2) | 0.0268 (6) | |
| C2 | −0.22273 (16) | 0.09570 (13) | 1.1505 (2) | 0.0307 (6) | |
| H2 | −0.2766 | 0.0938 | 1.1884 | 0.037* | |
| C3 | −0.18104 (16) | 0.15392 (14) | 1.1173 (2) | 0.0302 (6) | |
| H3 | −0.2003 | 0.2004 | 1.1272 | 0.036* | |
| C4 | −0.10181 (15) | 0.13291 (13) | 1.0637 (2) | 0.0267 (6) | |
| C5 | −0.03796 (16) | 0.17749 (12) | 1.0229 (2) | 0.0242 (5) | |
| C6 | −0.05259 (15) | 0.25455 (12) | 1.0355 (2) | 0.0254 (5) | |
| C7 | −0.10737 (17) | 0.29051 (14) | 0.9522 (2) | 0.0347 (6) | |
| H7 | −0.1365 | 0.2661 | 0.8845 | 0.042* | |
| C8 | −0.12023 (17) | 0.36174 (14) | 0.9664 (2) | 0.0353 (6) | |
| H8 | −0.1582 | 0.3854 | 0.9087 | 0.042* | |
| C9 | −0.07828 (16) | 0.39834 (13) | 1.0637 (2) | 0.0284 (6) | |
| C10 | −0.02294 (18) | 0.36346 (14) | 1.1466 (2) | 0.0361 (7) | |
| H10 | 0.0069 | 0.3880 | 1.2134 | 0.043* | |
| C11 | −0.01107 (18) | 0.29203 (13) | 1.1314 (2) | 0.0354 (6) | |
| H11 | 0.0269 | 0.2684 | 1.1892 | 0.042* | |
| C12 | −0.04960 (18) | 0.50778 (12) | 1.1669 (3) | 0.0341 (6) | |
| H12A | 0.0109 | 0.5097 | 1.1459 | 0.041* | |
| H12B | −0.0545 | 0.4855 | 1.2528 | 0.041* | |
| C13 | −0.08601 (17) | 0.58049 (13) | 1.1696 (3) | 0.0339 (6) | |
| H13A | −0.0896 | 0.5991 | 1.0802 | 0.041* | |
| H13B | −0.0476 | 0.6110 | 1.2214 | 0.041* | |
| C14 | −0.17338 (17) | 0.58243 (13) | 1.2270 (3) | 0.0337 (6) | |
| H14A | −0.2116 | 0.5524 | 1.1739 | 0.040* | |
| H14B | −0.1696 | 0.5623 | 1.3153 | 0.040* | |
| C15 | −0.21219 (17) | 0.65466 (14) | 1.2351 (3) | 0.0350 (6) | |
| H15A | −0.2249 | 0.6720 | 1.1463 | 0.042* | |
| H15B | −0.1707 | 0.6868 | 1.2765 | 0.042* | |
| C16 | −0.29271 (18) | 0.65544 (14) | 1.3115 (3) | 0.0383 (7) | |
| H16A | −0.3327 | 0.6213 | 1.2724 | 0.046* | |
| H16B | −0.2790 | 0.6399 | 1.4011 | 0.046* | |
| C17 | −0.3360 (2) | 0.72544 (17) | 1.3171 (3) | 0.0521 (8) | |
| H17A | −0.3525 | 0.7400 | 1.2280 | 0.062* | |
| H17B | −0.2954 | 0.7603 | 1.3527 | 0.062* | |
| C18 | −0.4137 (2) | 0.72520 (19) | 1.3994 (4) | 0.0679 (10) | |
| H18A | −0.4538 | 0.6902 | 1.3659 | 0.102* | |
| H18B | −0.4403 | 0.7713 | 1.3961 | 0.102* | |
| H18C | −0.3973 | 0.7139 | 1.4892 | 0.102* | |
| C19 | 0.04070 (16) | 0.15704 (12) | 0.9737 (2) | 0.0240 (5) | |
| C20 | 0.10418 (16) | 0.20316 (13) | 0.9264 (2) | 0.0291 (6) | |
| H20 | 0.1026 | 0.2526 | 0.9284 | 0.035* | |
| C21 | 0.16623 (16) | 0.16374 (13) | 0.8789 (2) | 0.0281 (6) | |
| H21 | 0.2160 | 0.1804 | 0.8402 | 0.034* | |
| C22 | 0.14361 (15) | 0.09178 (13) | 0.8972 (2) | 0.0253 (6) | |
| C23 | 0.19112 (15) | 0.03383 (14) | 0.8601 (2) | 0.0264 (6) | |
| C24 | 0.26926 (16) | 0.04802 (13) | 0.7844 (2) | 0.0284 (6) | |
| C25 | 0.26415 (17) | 0.04737 (15) | 0.6514 (3) | 0.0379 (7) | |
| H25 | 0.2115 | 0.0372 | 0.6092 | 0.045* | |
| C26 | 0.33407 (17) | 0.06126 (15) | 0.5767 (3) | 0.0397 (7) | |
| H26 | 0.3292 | 0.0597 | 0.4849 | 0.048* | |
| C27 | 0.41000 (17) | 0.07728 (13) | 0.6369 (3) | 0.0326 (6) | |
| C28 | 0.41643 (17) | 0.07760 (16) | 0.7708 (3) | 0.0428 (7) | |
| H28 | 0.4690 | 0.0880 | 0.8130 | 0.051* | |
| C29 | 0.34655 (17) | 0.06290 (15) | 0.8436 (3) | 0.0388 (7) | |
| H29 | 0.3519 | 0.0630 | 0.9354 | 0.047* | |
| C30 | 0.47894 (19) | 0.09010 (16) | 0.4349 (3) | 0.0432 (7) | |
| H30A | 0.4315 | 0.1190 | 0.4008 | 0.052* | |
| H30B | 0.4693 | 0.0414 | 0.4062 | 0.052* | |
| C31 | 0.56106 (19) | 0.11666 (15) | 0.3838 (3) | 0.0466 (8) | |
| H31A | 0.5638 | 0.1047 | 0.2907 | 0.056* | |
| H31B | 0.6084 | 0.0927 | 0.4298 | 0.056* | |
| C32 | 0.57217 (18) | 0.19524 (15) | 0.3996 (3) | 0.0431 (7) | |
| H32A | 0.5192 | 0.2186 | 0.3707 | 0.052* | |
| H32B | 0.5817 | 0.2059 | 0.4927 | 0.052* | |
| C33 | 0.64472 (18) | 0.22525 (15) | 0.3246 (3) | 0.0437 (7) | |
| H33A | 0.6986 | 0.2064 | 0.3608 | 0.052* | |
| H33B | 0.6390 | 0.2100 | 0.2331 | 0.052* | |
| C34 | 0.6478 (2) | 0.30437 (16) | 0.3290 (3) | 0.0500 (8) | |
| H34A | 0.5923 | 0.3228 | 0.2990 | 0.060* | |
| H34B | 0.6573 | 0.3192 | 0.4201 | 0.060* | |
| C35 | 0.7154 (2) | 0.33636 (16) | 0.2479 (4) | 0.0569 (9) | |
| H35A | 0.7076 | 0.3200 | 0.1575 | 0.068* | |
| H35B | 0.7713 | 0.3198 | 0.2805 | 0.068* | |
| C36 | 0.7149 (3) | 0.41504 (18) | 0.2490 (4) | 0.0837 (13) | |
| H36A | 0.6608 | 0.4319 | 0.2128 | 0.126* | |
| H36B | 0.7609 | 0.4325 | 0.1967 | 0.126* | |
| H36C | 0.7225 | 0.4317 | 0.3383 | 0.126* | |
| N1 | −0.09901 (12) | 0.06134 (10) | 1.06458 (18) | 0.0258 (5) | |
| N2 | 0.06710 (12) | 0.08969 (10) | 0.95571 (18) | 0.0236 (4) | |
| O1 | −0.09551 (11) | 0.46820 (9) | 1.07045 (16) | 0.0327 (4) | |
| O2 | 0.48278 (11) | 0.09324 (10) | 0.57368 (18) | 0.0400 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ag1 | 0.02106 (15) | 0.01858 (15) | 0.02898 (14) | 0.00032 (12) | 0.00751 (10) | 0.00036 (11) |
| C1 | 0.0219 (14) | 0.0264 (15) | 0.0324 (13) | 0.0016 (11) | 0.0069 (11) | −0.0005 (11) |
| C2 | 0.0256 (14) | 0.0273 (15) | 0.0399 (14) | 0.0000 (12) | 0.0114 (11) | −0.0009 (11) |
| C3 | 0.0270 (14) | 0.0256 (14) | 0.0386 (14) | 0.0045 (12) | 0.0089 (11) | −0.0017 (11) |
| C4 | 0.0245 (14) | 0.0254 (15) | 0.0303 (13) | 0.0028 (11) | 0.0025 (10) | −0.0006 (10) |
| C5 | 0.0265 (14) | 0.0195 (13) | 0.0267 (12) | 0.0012 (11) | 0.0036 (10) | −0.0009 (10) |
| C6 | 0.0243 (13) | 0.0210 (13) | 0.0313 (13) | 0.0003 (11) | 0.0064 (10) | 0.0012 (10) |
| C7 | 0.0372 (16) | 0.0267 (15) | 0.0399 (14) | 0.0015 (12) | −0.0061 (12) | −0.0058 (12) |
| C8 | 0.0391 (16) | 0.0251 (15) | 0.0411 (15) | 0.0078 (13) | −0.0090 (12) | 0.0004 (12) |
| C9 | 0.0327 (15) | 0.0202 (13) | 0.0326 (13) | 0.0030 (11) | 0.0063 (11) | 0.0010 (11) |
| C10 | 0.0457 (17) | 0.0268 (15) | 0.0355 (14) | 0.0032 (13) | −0.0059 (12) | −0.0079 (11) |
| C11 | 0.0447 (17) | 0.0249 (15) | 0.0361 (14) | 0.0097 (13) | −0.0060 (12) | −0.0010 (11) |
| C12 | 0.0394 (16) | 0.0235 (15) | 0.0394 (14) | 0.0003 (12) | 0.0022 (12) | −0.0067 (11) |
| C13 | 0.0379 (16) | 0.0238 (15) | 0.0404 (15) | 0.0003 (12) | 0.0055 (12) | −0.0036 (11) |
| C14 | 0.0385 (16) | 0.0241 (15) | 0.0387 (14) | −0.0030 (12) | 0.0059 (12) | −0.0013 (11) |
| C15 | 0.0376 (17) | 0.0255 (15) | 0.0423 (15) | −0.0006 (13) | 0.0102 (12) | −0.0029 (12) |
| C16 | 0.0392 (17) | 0.0338 (16) | 0.0422 (15) | −0.0006 (13) | 0.0069 (13) | −0.0012 (13) |
| C17 | 0.046 (2) | 0.0436 (19) | 0.067 (2) | 0.0084 (16) | 0.0201 (16) | −0.0013 (16) |
| C18 | 0.067 (3) | 0.059 (2) | 0.080 (2) | 0.015 (2) | 0.036 (2) | 0.0017 (19) |
| C19 | 0.0279 (14) | 0.0190 (13) | 0.0254 (12) | −0.0008 (11) | 0.0030 (10) | −0.0001 (10) |
| C20 | 0.0293 (15) | 0.0223 (14) | 0.0360 (13) | −0.0010 (12) | 0.0052 (11) | 0.0014 (11) |
| C21 | 0.0238 (14) | 0.0250 (14) | 0.0359 (14) | −0.0045 (11) | 0.0075 (11) | 0.0039 (11) |
| C22 | 0.0239 (14) | 0.0229 (14) | 0.0294 (12) | −0.0014 (11) | 0.0038 (10) | 0.0006 (10) |
| C23 | 0.0212 (14) | 0.0285 (15) | 0.0296 (13) | −0.0007 (12) | 0.0049 (10) | −0.0015 (11) |
| C24 | 0.0266 (14) | 0.0205 (14) | 0.0387 (14) | −0.0001 (11) | 0.0092 (11) | −0.0015 (11) |
| C25 | 0.0267 (15) | 0.0478 (18) | 0.0393 (15) | −0.0075 (13) | 0.0039 (12) | 0.0008 (13) |
| C26 | 0.0344 (16) | 0.0507 (19) | 0.0345 (14) | −0.0066 (14) | 0.0078 (12) | 0.0040 (13) |
| C27 | 0.0293 (15) | 0.0251 (15) | 0.0441 (15) | −0.0008 (12) | 0.0146 (12) | −0.0003 (12) |
| C28 | 0.0251 (15) | 0.055 (2) | 0.0480 (16) | −0.0101 (14) | 0.0058 (12) | −0.0047 (14) |
| C29 | 0.0316 (16) | 0.0503 (19) | 0.0350 (14) | −0.0076 (14) | 0.0072 (12) | −0.0063 (13) |
| C30 | 0.0437 (18) | 0.0407 (18) | 0.0463 (16) | −0.0043 (15) | 0.0211 (14) | 0.0019 (14) |
| C31 | 0.0451 (19) | 0.0381 (18) | 0.0581 (18) | 0.0015 (15) | 0.0265 (15) | 0.0034 (15) |
| C32 | 0.0376 (17) | 0.0376 (17) | 0.0551 (17) | 0.0015 (14) | 0.0189 (14) | 0.0007 (14) |
| C33 | 0.0329 (16) | 0.0355 (17) | 0.0637 (19) | 0.0010 (14) | 0.0175 (14) | 0.0060 (14) |
| C34 | 0.047 (2) | 0.0381 (18) | 0.066 (2) | −0.0011 (15) | 0.0148 (16) | 0.0048 (15) |
| C35 | 0.046 (2) | 0.0369 (19) | 0.088 (2) | −0.0063 (16) | 0.0157 (18) | 0.0155 (17) |
| C36 | 0.083 (3) | 0.040 (2) | 0.130 (4) | −0.014 (2) | 0.035 (3) | 0.011 (2) |
| N1 | 0.0238 (11) | 0.0196 (11) | 0.0346 (11) | 0.0014 (9) | 0.0092 (9) | 0.0005 (9) |
| N2 | 0.0214 (11) | 0.0199 (11) | 0.0300 (10) | 0.0016 (9) | 0.0071 (8) | 0.0000 (8) |
| O1 | 0.0418 (12) | 0.0206 (9) | 0.0355 (10) | 0.0054 (8) | −0.0016 (8) | −0.0037 (8) |
| O2 | 0.0299 (11) | 0.0412 (12) | 0.0499 (11) | −0.0050 (9) | 0.0179 (9) | 0.0035 (9) |
Geometric parameters (Å, °)
| Ag1—N2 | 2.0814 (19) | C18—H18B | 0.9800 |
| Ag1—N2i | 2.0815 (19) | C18—H18C | 0.9800 |
| Ag1—N1i | 2.0871 (19) | C19—N2 | 1.373 (3) |
| Ag1—N1 | 2.0872 (19) | C19—C20 | 1.436 (3) |
| C1—N1 | 1.367 (3) | C20—C21 | 1.345 (3) |
| C1—C23i | 1.417 (4) | C20—H20 | 0.9500 |
| C1—C2 | 1.435 (3) | C21—C22 | 1.441 (3) |
| C2—C3 | 1.348 (3) | C21—H21 | 0.9500 |
| C2—H2 | 0.9500 | C22—N2 | 1.371 (3) |
| C3—C4 | 1.445 (3) | C22—C23 | 1.403 (3) |
| C3—H3 | 0.9500 | C23—C1i | 1.417 (4) |
| C4—N1 | 1.374 (3) | C23—C24 | 1.509 (3) |
| C4—C5 | 1.400 (3) | C24—C25 | 1.373 (3) |
| C5—C19 | 1.416 (3) | C24—C29 | 1.382 (4) |
| C5—C6 | 1.503 (3) | C25—C26 | 1.395 (3) |
| C6—C11 | 1.376 (3) | C25—H25 | 0.9500 |
| C6—C7 | 1.387 (3) | C26—C27 | 1.372 (4) |
| C7—C8 | 1.390 (4) | C26—H26 | 0.9500 |
| C7—H7 | 0.9500 | C27—O2 | 1.377 (3) |
| C8—C9 | 1.380 (3) | C27—C28 | 1.384 (4) |
| C8—H8 | 0.9500 | C28—C29 | 1.386 (4) |
| C9—O1 | 1.370 (3) | C28—H28 | 0.9500 |
| C9—C10 | 1.380 (3) | C29—H29 | 0.9500 |
| C10—C11 | 1.393 (4) | C30—O2 | 1.435 (3) |
| C10—H10 | 0.9500 | C30—C31 | 1.508 (4) |
| C11—H11 | 0.9500 | C30—H30A | 0.9900 |
| C12—O1 | 1.434 (3) | C30—H30B | 0.9900 |
| C12—C13 | 1.510 (3) | C31—C32 | 1.526 (4) |
| C12—H12A | 0.9900 | C31—H31A | 0.9900 |
| C12—H12B | 0.9900 | C31—H31B | 0.9900 |
| C13—C14 | 1.523 (4) | C32—C33 | 1.519 (4) |
| C13—H13A | 0.9900 | C32—H32A | 0.9900 |
| C13—H13B | 0.9900 | C32—H32B | 0.9900 |
| C14—C15 | 1.520 (3) | C33—C34 | 1.520 (4) |
| C14—H14A | 0.9900 | C33—H33A | 0.9900 |
| C14—H14B | 0.9900 | C33—H33B | 0.9900 |
| C15—C16 | 1.521 (4) | C34—C35 | 1.510 (4) |
| C15—H15A | 0.9900 | C34—H34A | 0.9900 |
| C15—H15B | 0.9900 | C34—H34B | 0.9900 |
| C16—C17 | 1.510 (4) | C35—C36 | 1.510 (4) |
| C16—H16A | 0.9900 | C35—H35A | 0.9900 |
| C16—H16B | 0.9900 | C35—H35B | 0.9900 |
| C17—C18 | 1.518 (4) | C36—H36A | 0.9800 |
| C17—H17A | 0.9900 | C36—H36B | 0.9800 |
| C17—H17B | 0.9900 | C36—H36C | 0.9800 |
| C18—H18A | 0.9800 | ||
| N2—Ag1—N2i | 180.0 | N2—C19—C20 | 108.3 (2) |
| N2—Ag1—N1i | 90.12 (7) | C5—C19—C20 | 125.8 (2) |
| N2i—Ag1—N1i | 89.88 (7) | C21—C20—C19 | 107.8 (2) |
| N2—Ag1—N1 | 89.88 (7) | C21—C20—H20 | 126.1 |
| N2i—Ag1—N1 | 90.12 (7) | C19—C20—H20 | 126.1 |
| N1i—Ag1—N1 | 180.00 (7) | C20—C21—C22 | 107.6 (2) |
| N1—C1—C23i | 125.8 (2) | C20—C21—H21 | 126.2 |
| N1—C1—C2 | 108.5 (2) | C22—C21—H21 | 126.2 |
| C23i—C1—C2 | 125.8 (2) | N2—C22—C23 | 125.9 (2) |
| C3—C2—C1 | 107.7 (2) | N2—C22—C21 | 108.2 (2) |
| C3—C2—H2 | 126.2 | C23—C22—C21 | 125.9 (2) |
| C1—C2—H2 | 126.2 | C22—C23—C1i | 126.5 (2) |
| C2—C3—C4 | 107.7 (2) | C22—C23—C24 | 117.0 (2) |
| C2—C3—H3 | 126.1 | C1i—C23—C24 | 116.5 (2) |
| C4—C3—H3 | 126.1 | C25—C24—C29 | 118.0 (2) |
| N1—C4—C5 | 126.1 (2) | C25—C24—C23 | 119.5 (2) |
| N1—C4—C3 | 107.7 (2) | C29—C24—C23 | 122.6 (2) |
| C5—C4—C3 | 126.1 (2) | C24—C25—C26 | 121.8 (3) |
| C4—C5—C19 | 126.2 (2) | C24—C25—H25 | 119.1 |
| C4—C5—C6 | 117.4 (2) | C26—C25—H25 | 119.1 |
| C19—C5—C6 | 116.3 (2) | C27—C26—C25 | 119.5 (2) |
| C11—C6—C7 | 117.6 (2) | C27—C26—H26 | 120.2 |
| C11—C6—C5 | 120.3 (2) | C25—C26—H26 | 120.2 |
| C7—C6—C5 | 122.1 (2) | O2—C27—C26 | 124.8 (2) |
| C6—C7—C8 | 121.0 (2) | O2—C27—C28 | 115.8 (2) |
| C6—C7—H7 | 119.5 | C26—C27—C28 | 119.4 (2) |
| C8—C7—H7 | 119.5 | C27—C28—C29 | 120.3 (3) |
| C9—C8—C7 | 120.5 (2) | C27—C28—H28 | 119.8 |
| C9—C8—H8 | 119.7 | C29—C28—H28 | 119.8 |
| C7—C8—H8 | 119.7 | C28—C29—C24 | 120.9 (2) |
| O1—C9—C8 | 116.3 (2) | C28—C29—H29 | 119.5 |
| O1—C9—C10 | 124.5 (2) | C24—C29—H29 | 119.5 |
| C8—C9—C10 | 119.2 (2) | O2—C30—C31 | 108.9 (2) |
| C9—C10—C11 | 119.5 (2) | O2—C30—H30A | 109.9 |
| C9—C10—H10 | 120.2 | C31—C30—H30A | 109.9 |
| C11—C10—H10 | 120.2 | O2—C30—H30B | 109.9 |
| C6—C11—C10 | 122.1 (2) | C31—C30—H30B | 109.9 |
| C6—C11—H11 | 118.9 | H30A—C30—H30B | 108.3 |
| C10—C11—H11 | 118.9 | C30—C31—C32 | 113.2 (2) |
| O1—C12—C13 | 108.4 (2) | C30—C31—H31A | 108.9 |
| O1—C12—H12A | 110.0 | C32—C31—H31A | 108.9 |
| C13—C12—H12A | 110.0 | C30—C31—H31B | 108.9 |
| O1—C12—H12B | 110.0 | C32—C31—H31B | 108.9 |
| C13—C12—H12B | 110.0 | H31A—C31—H31B | 107.7 |
| H12A—C12—H12B | 108.4 | C33—C32—C31 | 114.0 (2) |
| C12—C13—C14 | 112.5 (2) | C33—C32—H32A | 108.8 |
| C12—C13—H13A | 109.1 | C31—C32—H32A | 108.8 |
| C14—C13—H13A | 109.1 | C33—C32—H32B | 108.8 |
| C12—C13—H13B | 109.1 | C31—C32—H32B | 108.8 |
| C14—C13—H13B | 109.1 | H32A—C32—H32B | 107.7 |
| H13A—C13—H13B | 107.8 | C34—C33—C32 | 112.8 (2) |
| C15—C14—C13 | 114.7 (2) | C34—C33—H33A | 109.0 |
| C15—C14—H14A | 108.6 | C32—C33—H33A | 109.0 |
| C13—C14—H14A | 108.6 | C34—C33—H33B | 109.0 |
| C15—C14—H14B | 108.6 | C32—C33—H33B | 109.0 |
| C13—C14—H14B | 108.6 | H33A—C33—H33B | 107.8 |
| H14A—C14—H14B | 107.6 | C35—C34—C33 | 114.3 (3) |
| C14—C15—C16 | 112.6 (2) | C35—C34—H34A | 108.7 |
| C14—C15—H15A | 109.1 | C33—C34—H34A | 108.7 |
| C16—C15—H15A | 109.1 | C35—C34—H34B | 108.7 |
| C14—C15—H15B | 109.1 | C33—C34—H34B | 108.7 |
| C16—C15—H15B | 109.1 | H34A—C34—H34B | 107.6 |
| H15A—C15—H15B | 107.8 | C34—C35—C36 | 113.5 (3) |
| C17—C16—C15 | 114.7 (2) | C34—C35—H35A | 108.9 |
| C17—C16—H16A | 108.6 | C36—C35—H35A | 108.9 |
| C15—C16—H16A | 108.6 | C34—C35—H35B | 108.9 |
| C17—C16—H16B | 108.6 | C36—C35—H35B | 108.9 |
| C15—C16—H16B | 108.6 | H35A—C35—H35B | 107.7 |
| H16A—C16—H16B | 107.6 | C35—C36—H36A | 109.5 |
| C16—C17—C18 | 113.3 (3) | C35—C36—H36B | 109.5 |
| C16—C17—H17A | 108.9 | H36A—C36—H36B | 109.5 |
| C18—C17—H17A | 108.9 | C35—C36—H36C | 109.5 |
| C16—C17—H17B | 108.9 | H36A—C36—H36C | 109.5 |
| C18—C17—H17B | 108.9 | H36B—C36—H36C | 109.5 |
| H17A—C17—H17B | 107.7 | C1—N1—C4 | 108.4 (2) |
| C17—C18—H18A | 109.5 | C1—N1—Ag1 | 125.72 (17) |
| C17—C18—H18B | 109.5 | C4—N1—Ag1 | 125.88 (16) |
| H18A—C18—H18B | 109.5 | C19—N2—C22 | 108.01 (19) |
| C17—C18—H18C | 109.5 | C19—N2—Ag1 | 126.08 (15) |
| H18A—C18—H18C | 109.5 | C22—N2—Ag1 | 125.83 (16) |
| H18B—C18—H18C | 109.5 | C9—O1—C12 | 117.12 (19) |
| N2—C19—C5 | 125.8 (2) | C27—O2—C30 | 117.0 (2) |
Symmetry codes: (i) −x, −y, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BG2416).
References
- Bruker (2002). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Fu, B., Yu, H.-C, Huang, J.-W., Zhao, P., Liu, J. & Ji, L.-N. (2009). J. Mol. Catal. A Chem. 298, 74–80.
- Jurow, M., Schuckman, A. E., Batteas, J. D. & Drain, C. M. (2010). Coord. Chem. Rev. 254, 2297–2310. [DOI] [PMC free article] [PubMed]
- Scheidt, W. R., Mondal, J. U., Eigenbrot, C. W., Adler, A., Radonvich, L. J. & Hoard, J. L. (1986). Inorg. Chem. 25, 795–799.
- Sheldrick, G. M. (2004). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Taniguchi, M. & Lindsey, J. S. (2010). Tetrahedron, 66, 5549–5565.
- Xu, Y.-J., Yang, X.-X., Cao, H. & Zhao, H.-B. (2007). Acta Cryst. E63, m1437.
- Zenkevich, E. I., Willert, A., Bachilo, S. M., Rempel, U., Kilin, D. S., Shulga, A. M. & von Borczyskowski, C. (2001). Mater. Sci. Eng. C, 18, 99–111.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S160053681103385X/bg2416sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S160053681103385X/bg2416Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



