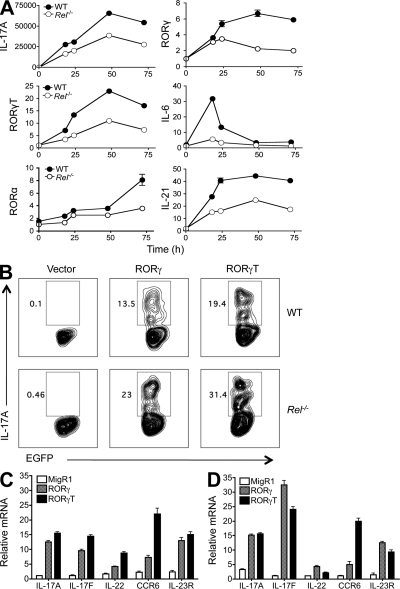
Figure 2.
RORγ and RORγT expression is significantly reduced in c-Rel–deficient T cells, and RORγ reconstitution rescues the Th17 defect. (A) Purified naive CD4+ splenic T cells from 6-wk-old WT (n = 5) and Rel−/− (n = 5) mice were cultured under Th17 differentiation condition as described in Materials and methods. Cells were collected at different time points as indicated, and total RNA was extracted. The mRNA levels of the indicated genes were determined by real-time RT-PCR and normalized to GAPDH. Error bars indicate the SDs of the means, which are visible only if they are bigger than the diameters of the symbols. (B) Purified splenic CD4+ T cells from WT (n = 5) and Rel−/− mice (n = 5) were cultured under Th0-inducing conditions in the presence of anti–IL-4 and anti–IFN-γ for 24 h. Cells were then infected with recombinant retroviruses that carry EGFP (Vector as a control), RORγ-EGFP (RORγ), or RORγt-EGFP (RORγt) cDNA as described in Materials and methods. 4 d later, cells were restimulated with PMA and ionomycin and stained intracellularly with anti–IL-17A, and analyzed by flow cytometry. Data shown are for gated EGFP+ cells. Results are representative of two independent experiments. (C and D) Splenic CD4+ T cells were isolated from 6-wk-old WT (C) and Rel−/− (D) mice (n = 3), and infected with recombinant retroviruses that carry EGFP (vector control), RORγ-EGFP, or RORγT-EGFP cDNA, as described in B. 4 d later, EGFP+ cells were sorted, total RNA was extracted, and mRNA levels of the indicated genes were determined by real-time RT-PCR. Data shown are fold increases over vector-treated groups, and are means and SD of triplicate cultures. Results are representative of two independent experiments.