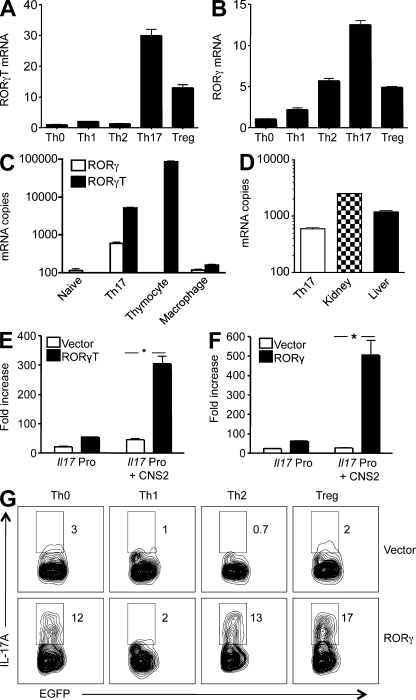
Figure 3.
RORγT and RORγ activate the Il17 promoter and drive Th17 differentiation. (A and B) Purified CD4+ T cells were cultured under conditions that induce Th0, Th1, Th2, Th17, and T reg cells as described in Materials and methods. 3 d later, cells were harvested and restimulated with PMA and ionomycin for an additional 3 h. The RORγT (A) and RORγ (B) mRNA levels were determined by real-time RT-PCR, and are presented as fold increases over that of the Th0 cells. Data shown are representative of two independent experiments. (C and D) Total RNA was extracted from naive CD4+ T cells (Naive), Th17 cells, thymocytes, peritoneal macrophages, kidney, and liver of B6 mice as indicated. The mRNA copy numbers of RORγ and RORγT were determined by real-time RT-PCR using the respective cDNAs as standards. (E and F) EL4 T cells were transiently transfected with luciferase constructs of the murine Il17a promoter (Il17 Pro) or Il17a promoter linked to its CNS2 (Il17 Pro + CNS2), together with an expression vector for full-length RORγT (C) or RORγ (D), or the empty vector as indicated. After 24 h, cells were treated with PMA and ionomycin for 4 h, and the luciferase activities measured. The promoter activity is presented as fold increase over cells transfected with the empty vector. To normalize the transfection efficiency across samples, the Renilla luciferase expression vector pRLTK was used as an internal control. Data are representative of two independent experiments. *, P < 0.01. (G) Purified CD4+ T cells were cultured under conditions that induce Th0, Th1, Th2, Th17, and T reg cells for 24 h, and then infected with retroviruses that encode EGFP (Vector) or RORγ and EGFP (RORγ) as described in Materials and methods. 4 d later, cells were restimulated with PMA and ionomycin in the presence of GolgiStop for 4 h, stained intracellularly with antibodies to IL-17A, and analyzed by flow cytometry. Data shown are for gated EGFP+ cells. The experiment was repeated twice with similar results.