Figure 4.
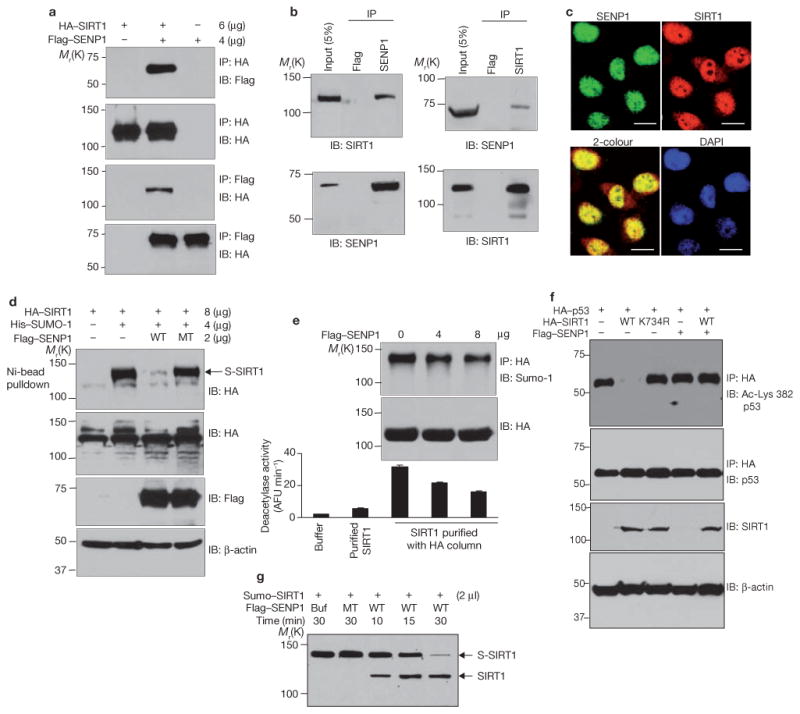
Desumoylation of SIRT1 by SENP1 reduces its ability to deacetylate p53. (a) H1299 cells were cotransfected with HA–SIRT1 and Flag–SENP1. HA and Flag immunoprecipitates were immunoblotted with antibodies against Flag or HA. (b) Extracts of H1299 cells were immunoprecipitated with antibodies against Flag, SENP1 or SIRT1 and immunoblotted with antibodies as indicated. (c) H1299 cells were fixed and immunostained with antibodies against SENP1 or SIRT1. Colocalization was detected by confocal fluorescence microscopy. The scale bars represent 20 μm. (d) Cells were cotransfected with HA–SIRT1, His–Sumo-1 and Flag–SENP1, as indicated. Sumoylated HA–SIRT1 was isolated by Ni-NTA agarose and detected by western blotting with antibodies against HA. Amounts of SIRT1 (anti-HA), wild-type and mutant (MT) SENP1 (anti-Flag), and β-actin were determined by western blotting. (e) Cells were cotransfected with HA–SIRT1 and SENP1. HA–SIRT1 was purified from cell extracts on an anti-HA conjugated affinity column, and its activity was determined by a fluorometric assay system. Commercially obtained recombinant SIRT1 protein is included in the assay as a reference (lane 2). Each data point is the average of triplicate samples (n = 3) analysed in parallel. The error bars represent s.d. (f) Cells were cotransfected with HA–p53, Flag–SENP1 and wild-type Flag–SIRT1 or Flag–SIRT1K734R. Anti-HA immunoprecipitates were immunoblotted with antibodies against p53 (total p53) or with antibodies against p53 acetylated at Lys 382. Cell extracts were immunoblotted with antibodies against HA or β-actin. (g) SIRT1 conjugated to His–SUMO-1 was isolated from HEK 293 cells using the μMACS His-tagged protein isolation kit and incubated with wild-type and inactive mutant SENP1 produced in vitro. Sumoylated and desumoylated SIRT1 were detected by immunoblotting with antibodies against SIRT1. Uncropped images of the scans are shown in the Supplementary Information, Fig. S1.
