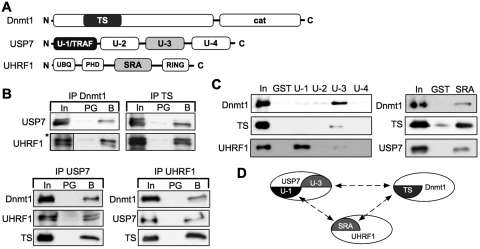
Figure 2.
Dnmt1, UHRF1 and USP7 form a trimeric complex. (A) Schematic representation of the domains of Dnmt1, USP7 and UHRF1 (proteins and domains are not in scale). TS (Targeting domain, amino acid 316–601), cat (C-terminal catalytic domain of Dnmt1). U-1 (TRAF domain, amino acid 1–215), U-2 (catalytic domain, amino acid 212–561), U-3 (C-terminal domain, amino acid 561–916), U-4 (C-terminus, amino acid 913–1102). UBQ (ubiquitin-like domain), PHD (plant homeodomain domain), SRA (SET-Ring finger associated domain, amino acid 435–586), RING (Ring finger domain). (B) Co-immunoprecipitation assay to determine the interaction of recombinant proteins with protein-specific antibodies. Detection of co-precipitated proteins via western blot. Input (In, 1.0%), protein G sepharose (PG, 20%), ‘specific beads’ (B, 20%), antibodies used for immunodetection are indicated. The Dnmt1-specific antibody DNM-2C1 recognizes the TS-domain and was used for IP and WB. (Asterisks) Five times shorter exposure of the input signal of UHRF1. (C) GST pull-down assay interaction analysis, using GST, the indicated USP7-domains and the SRA domain fused to GST with Dnmt1, TS-domain, USP7 and UHRF1. The bound proteins were separated and plotted with the respective antibodies. Input (In, 20%); GST or GST-fusion domains (25%). (D) Schematic overview of the protein interactions and protein-domains involved.