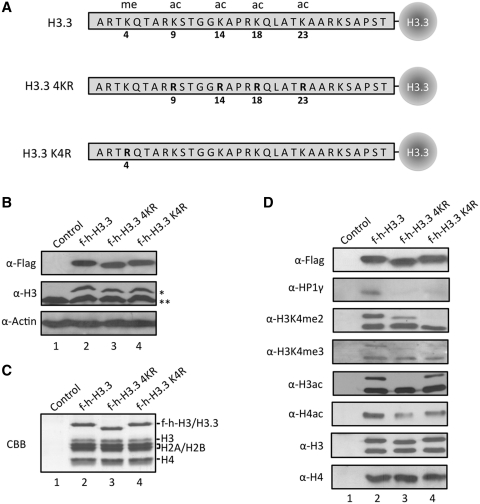
Figure 5.
Preferential binding of HP1γ to H3.3 containing mononucleosomes with active modifications. (A) Schematic diagrams of wild type (H3.3), acetylation site-mutated (H3.3 4KR) and K4-mutated (H3.3 K4R) H3.3. Four acetylatable lysine residues (K9, K14, K18 and K23) and one methylatable lysine residue (K4) are mutated to arginine. (B) HeLa cells were transfected with control (lane 1), wild type (lane 2) and mutant H3.3 (lane 3 and 4) expression plasmids for 48 h, and the expression levels of ectopic H3.3 were confirmed by western blotting. (C) Mononucleosomes containing wild type and mutant H3.3 were immunoprecipitated from total mononucleosomes, essentially following the procedure employed in Figure 1. Histone compositions of the purified nucleosomes were analyzed by 15% SDS–PAGE followed by Coomassie staining. (D) H3.3 mononucleosomes were purified as in Supplementary Figure S1A, and analyzed by western blotting using antibodies that recognize H3 acetylation (α-H3ac), H4 acetylation (α-H4ac), H3K4 dimethylation (α-H3K4me2), H3K4 trimethylation (α-H3K4me3), H3 (α-H3), H4 (α-H4) and HP1γ (α-HP1γ). Asterisk indicates ectopic f-h-H3 or f-h-H3.3 proteins and double asterisks indicate endogenous H3 or H3.3 proteins.