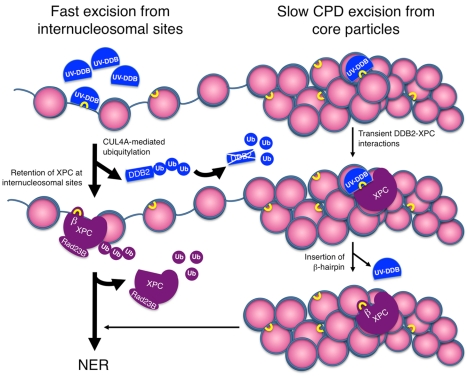
Figure 7. Novel regulatory principle in DNA repair.
Left, ubiquitin-dependent prioritization of DNA repair to internucleosomal sites. The preferential UV-DDB accumulation on internucleosomal DNA leads to ubiquitylation of the XPC partner by CUL4A ligase. This modification promotes the XPC retention at internucleosomal sites, thus reducing its association with nucleosome core particles. The implementation of this ubiquitin code is required for the fast initial excision of UV lesions from internucleosomal DNA. Concomitantly ubiquitylated DDB2 is degraded, but XPC protein is protected from proteasome activity by RAD23B [47]. Right, ubiquitin-independent priming platform. UV-DDB undergoes very transient interactions with the TGD and BHD1 motifs of XPC, thereby facilitating the insertion of a β-hairpin subdomain, to hand over the substrate to the downstream NER process. This ubiquitin-independent substrate handover is required, regardless of nucleosome localization, for the excision of CPDs that on their own induce minimal distortions of the DNA duplex and, hence, are not recognizable by XPC alone. Ub, ubiquitin; β, β-hairpin of XPC. UV lesions are indicated with yellow brackets.