Figure 2.
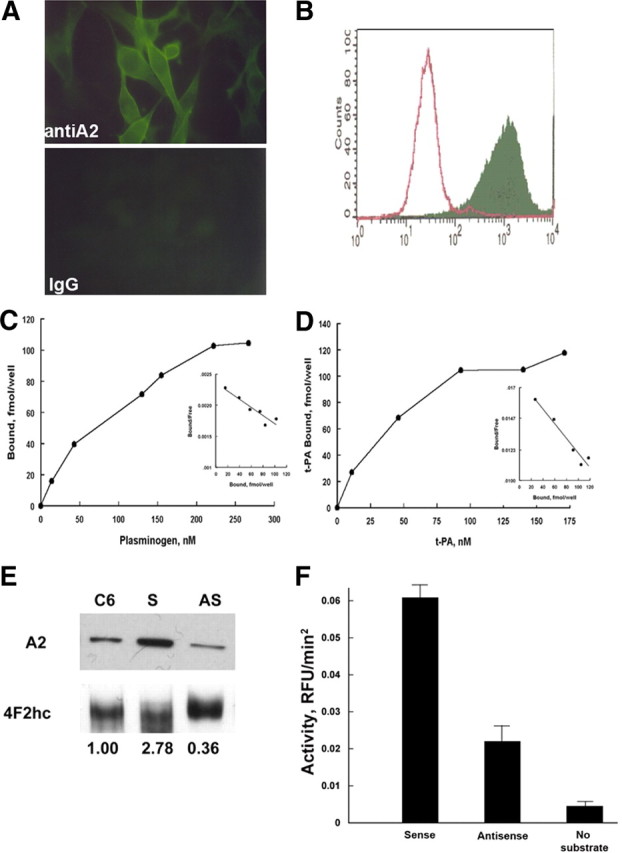
C6 glioma cells express annexin A2. A, Immunofluorescence. C6 glioma cells, propagated on chamber slides, were fixed in methanol (5 min, −20°C), blocked with 0.2% BSA in PBS (10 min, 21°C), and incubated with either mouse monoclonal anti-A2 (Zymed Laboratories; 1:100, 72 h, 4°C; top panel) or non-immune isotype-matched IgG (Zymed Laboratories; 1:100, 72 h, 4°C; bottom panel). After two washes in PBS, primary antibody reactivity was detected with FITC-labeled goat anti-mouse IgG (1:250, 60 min, 21°C). B, Flow cytometric analyses. C6 cells were incubated with polyclonal pre-immune (open peak) or anti–annexin A2 IgG (solid peak) (70 μg/ml, 4°C, 60 min) followed by fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (30 μg/ml, 4°C, 30 min; Cappel Laboratories), fixed in 2% paraformaldehyde (2 min, 21°C), and analyzed in a BD Biosciences flow cytometer (FACS Calibur). C6 glioma cells propagated in 24-well tissue culture plates were incubated with [125I]plasminogen (24,900 cpm/pmol) (C) or [125I]t-PA (503,000 cpm/pmol) in HBS containing 3 mm CaCl2, 1 mm MgCl2, and 5 mg/dl BSA (200 μl/well, 4°C, 60 min) over a range of concentrations (0–267 and 0–174 nm, respectively) (D). After sampling unbound ligand, cells were washed and solubilized, and bound radioactivity was counted. Scatchard analyses were performed as described previously (insets). E, Characterization of annexin A2 expression in stably transfected C6/LacZ glioma cells. Whole-cell lysates from annexin A2 sense- and antisense-transfected C6/LacZ cells were immunoblotted with monoclonal anti-annexin A2 IgG or polyclonal anti-4F2hc (21°C, 60 min) (Jacovina et al., 2001), using enhanced chemiluminescence (GE Healthcare). Annexin A2 expression was estimated after normalizing to the 4F2hc loading control using the Sigma gel scanning program (Jandel Scientific, version 1.0). F, Plasmin generation. Transfected C6/LacZ cells were incubated with plasminogen and t-PA, and plasmin generation was estimated as AFC-81 hydrolysis.
