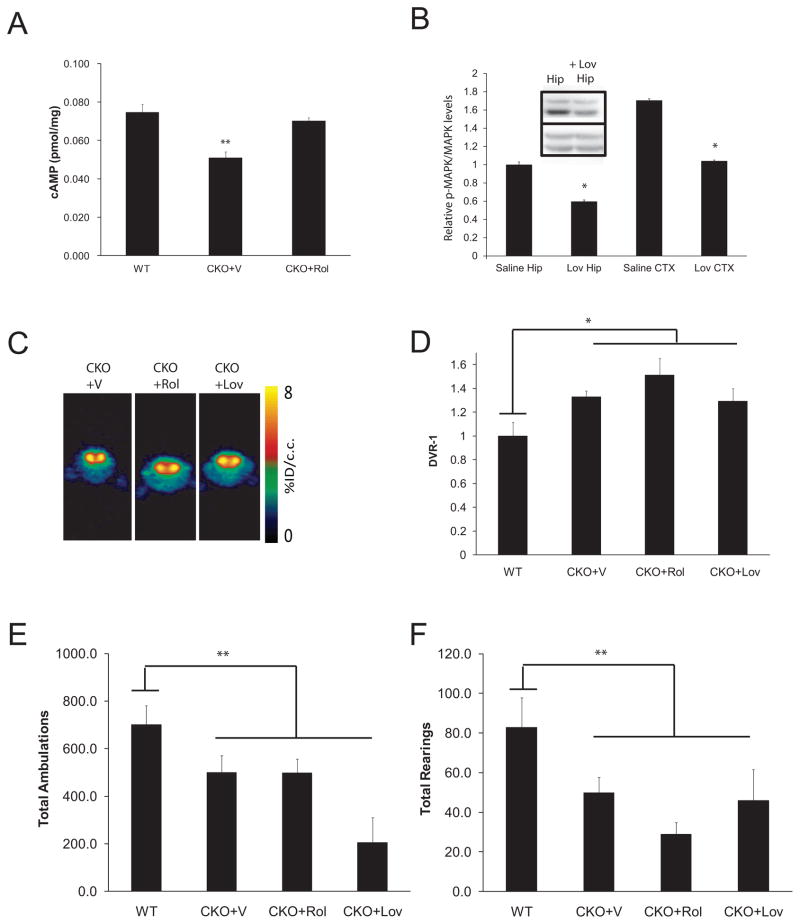
Fig. 3. Biologically-based neurofibromin therapies did not correct the DA defect or attentional deficit in CKO mice.
(A) Rolipram (CKO+Rol; 5mg/kg/day × 2 weeks) treatment restored cAMP levels in the striatum of 3-month-old mice. (B) Lovastatin (Lov; 10mg/kg i.p.) reduced MAPK activation (p-MAPK) in the cortex (CTX) and hippocampus (Hip) of CKO mice following normalization to total MAPK expression (p=.001; N=3). All fold changes (relative pMAPK/MAPK levels) are relative to saline-treated (vehicle; V) hippocampal levels. Inset shows a representative Western blot for p-MAPK and MAPK in the hippocampus (Hip) following saline and Lovastatin administration. (C) Representative [11C]-raclopride transverse micro-PET images (summed across 5–60 minutes) of CKO mice at baseline (vehicle-treated; V) and following Rolipram (Rol) and Lovastatin (Lov) treatment. The colorscale bar indicates the normalized peak uptake (percent injected dose per cubic centimeter tissue; %ID/cc). (D) Neither Rolipram nor Lovastatin reduced striatal [11C]-raclopride binding in CKO mice (N=8, N=4). During a 1h exploration of a novel environment, total ambulations (E) and total rearings (F) show no improvement in CKO mice following Rolipram or Lovastatin treatment. All mice used for the PET imaging experiments also underwent behavioral testing. Additional independently-generated WT and CKO mice, which did not undergo PET imaging, were included in the behavioral experiments.