Figure 1.
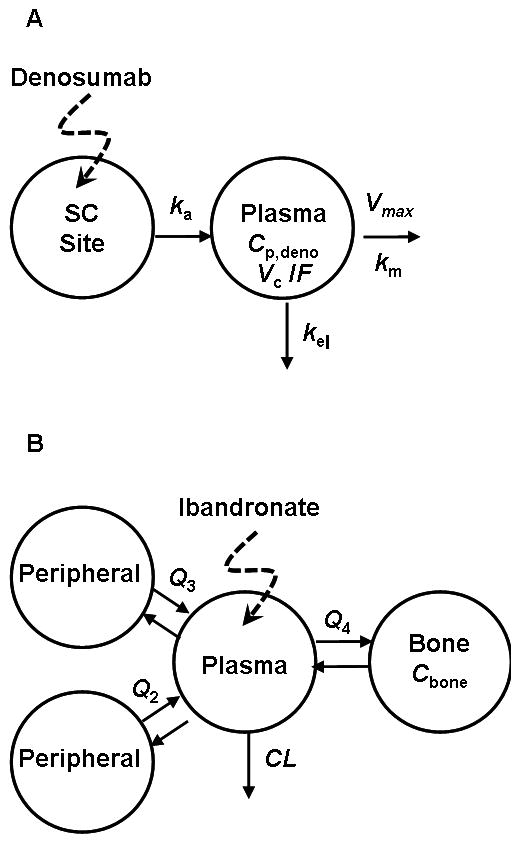
Pharmacokinetic models for denosumab (A) and ibandronate (B). For (A), drug administered subcutaneously is absorbed (ka) into the plasma compartment (Cp, Vc) and undergoes linear (kel) and nonlinear (Vmax, Km) elimination. Model shown in (B) is a linear 4-compartment model with a bone-specific site originally described by Pillai et al. [8].
