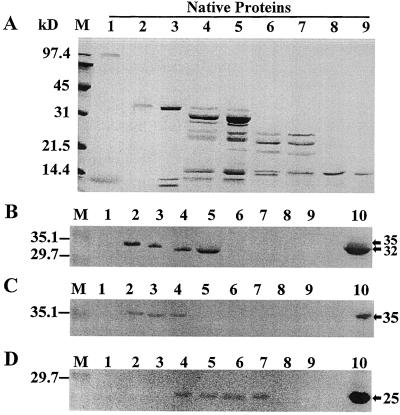
Figure 3.
Examination of native apoplastic proteins by SDS-PAGE and immunoblotting. A, Individual cold-acclimated apoplastic proteins were eluted from the native gel shown in Figure 1, denatured, separated by 15% SDS-PAGE, and stained with Coomassie brilliant blue R-250. Equal amounts of protein (5 μg) were loaded on each lane. Lanes 1 to 9, NP1 to NP9. Low-range prestained SDS-PAGE molecular-mass standards from Bio-Rad are shown on the left (lanes M). SDS-PAGE gels loaded with equal amounts (1 μg per lane) of individual native apoplastic proteins and with crude cold-acclimated apoplastic extract in lanes 10 were blotted and probed with anti-GLP antiserum (B), anti-CLP antiserum (C), and anti-TLP antiserum (D). Positive immunodetection of polypeptides in apoplastic extracts is indicated by arrows on the right.