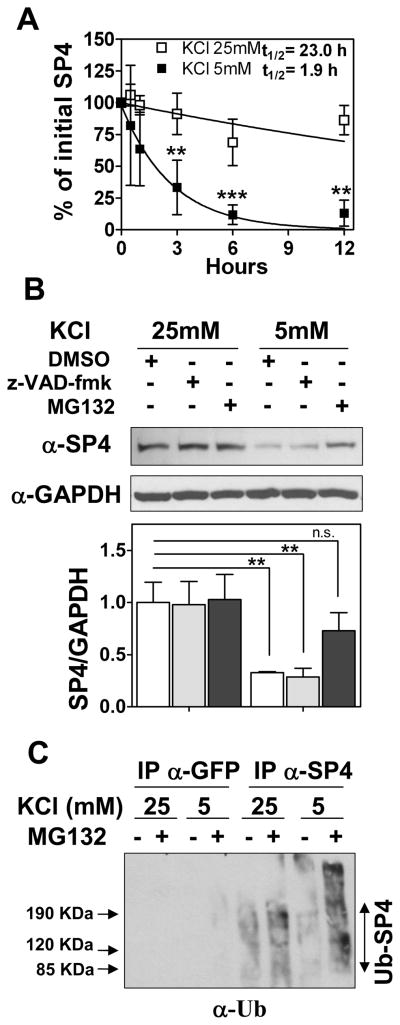
Fig. 4.
SP4 is polyubiquitinated and degraded via the proteasome in non-depolarizing conditions. (A) Cerebellar granule neurons were incubated in media containing serum and 50 μg/ml cycloheximide and 25 mM (open symbols) or 5 mM (closed symbols) KCl. Cell lysates were prepared at the indicated times and immunoblotted with anti-SP4 antisera. Quantitation was performed by densitometry and half-life (log10 50%) of SP4 was estimated by adjusting the percentage of initial SP4 percentage values to an exponential decay curve. Values represent the mean ± standard deviation of four independent experiments. Statistical analysis was performed using ANOVA and Tukey post-hoc test (***p < 0.001; **p < 0.01). (B) Granule neurons were exposed to control vehicle (DMSO), 100 μM MG-132 (proteasome inhibitor), or 80 μM z-VAD-fmk (broad range caspase inhibitor) one hour before and after changing into a culture medium with 25 mM or 5 mM KCl in the absence of serum. After one hour of incubation, cells were lysed and protein extracts were immunoblotted with antibody against SP4 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Values represent mean ± standard deviation of three independent experiments. Statistical analysis was performed using two-tailed t-test (**p < 0.01; n.s = not significant). (C) Granule neurons were incubated and treated with and without 10 μM MG-132 as in (B). Cell lysates were subjected to immunoprecipitation with antisera against SP4 or GFP. Immunoprecipitates were probed with an antibody against ubiquitin (α-Ub). An increase in high molecular weight species in the presence of MG132, particularly in low KCl, was also detected in anti-SP4 immunoblots (data not shown).