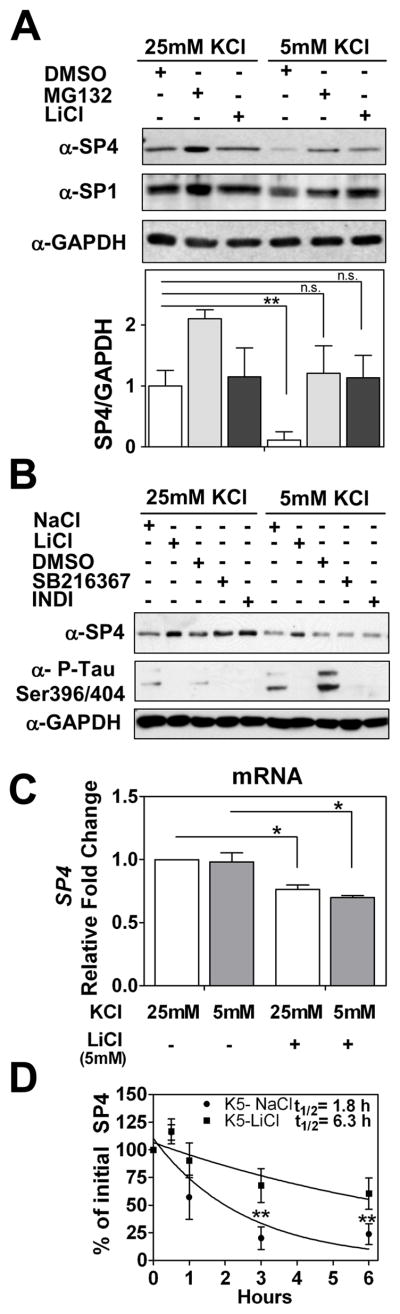
Fig. 5.
Lithium stabilized SP4 protein levels in non-depolarizing conditions through a GSK-3 independent mechanism. (A) Cerebellar granule neurons at 7 days in vitro (DIV-7) were treated with 100 μM MG132, 5 mM LiCl, or DMSO before and after switching cell medium to 25 mM or 5 mM KCl with serum. Cell lysates were analysed with antisera against SP4, SP1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). SP4 levels were analyzed by densitometry and normalized to GAPDH levels. Values represent mean ± standard deviation of three independent experiments. Statistical analysis was performed using two-tailed t-test (**p < 0.01; n.s. = not significant). (B) Granule neurons at DIV-7 were incubated with 6 μM SB216367, 5 μM Indirubin-3′-oxime (INDI), DMSO vehicle, 5 mM NaCl or 5 mM LiCl for five hours prior to and one hour after switching cells into media containing serum and 25 mM or 5 mM KCl. Extracts were immunoblotted with antibodies against SP4, GAPDH, or the GSK-3 substrate Tau phospho Ser396/Ser404 (PHF-1). (C) Granule neurons at DIV-7 were incubated with 5 mM LiCl or 5 mM NaCl for five hours before and one hour after switching into medium containing serum and 25 or 5 mM KCl for one hour. mRNA was obtained and quantitative RT-PCR reactions for SP4 and GAPDH were performed. Data represents mean ± standard deviation of two independent reactions performed in four biological repeats. Statistical analysis was performed using one-tailed t-test (*p < 0.05; n.s. = not significant). (D) Granule neurons were incubated with 5 mM LiCl or 5 mM NaCl for five hours and then switched into medium containing serum, 50 μg/ml cycloheximide, and 25 or 5 mM KCl for the indicated times. Immunoblot analysis were performed as described for Panel A. Half-life (log10 50%) of SP4 was estimated by adjusting the logarithm of initial SP4 percentage values to a linear regression. Values represent the mean ± standard deviation of four independent experiments. Statistical analysis was performed using ANOVA and Tukey post-hoc test (**p < 0.01)