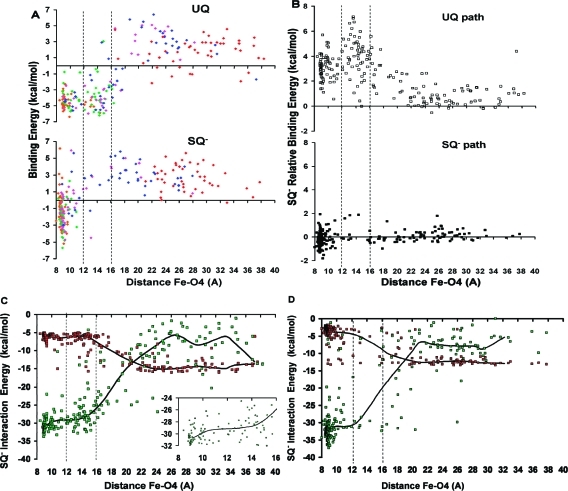
Figure 8.
Energies calculated with the MCCE implicit solvent method plotted against the distance between Fe2+ and the quinone carbonyl O4 atom along the SMD unbinding trajectories. Vertical dashed lines depict the suggested transition-state region where few SQ– headgroups are found (Figure 5A). (A) Binding free energy for UQ and SQ–. The color of each point indicates the SMD time point of the structure used for the calculation: orange (2000 ps), green (4800 ps), magenta (5800 ps), blue (6400 ps), and red (8000 ps) (Figure 4). (B) Relative binding energy of SQ– with respect to UQ (ΔGbind,SQ– – ΔGbind,UQ) in structures obtained from both the UQ and SQ– unbinding paths. Positive values indicate that SQ– is less stable than UQ in the regions of the protein away from the QA site. (C and D) SQ– total protein interaction (green) and implicit solvation energy (red). These energies are calculated along the UQ (C) and SQ– (D) exit pathways. The thick black lines plot the median energy at ≈2 Å intervals as a guide to the eye. The inset in (C) magnifies the transition-state region where SQ– loses stabilizing interactions with the protein.