Abstract
Background/Aim
Total-body irradiation (TBI) doses in the range of 2–8 Gy are associated with a drop in peripheral blood counts, decreased bone marrow cellularity, and hematopoietic syndrome. Radiation mitigators must be safe for individuals likely to recover spontaneously.
Materials and Methods
Female C57BL/6HNsd mice exposed to 9.0 and 9.15 Gy TBI, received intraperitoneal (10 mg/kg) JP4-039, a novel radiation mitigator, 24 hours after irradiation and were followed for hematopoietic recovery.
Results
Irradiated mice showed reduced peripheral blood lymphocytes and neutrophils and bone marrow cellularity at day 5. Serum electrolytes, liver and renal function tests showed no deleterious effect of JP4-039-after irradiation, and no reduction in survival compared to irradiated controls. Marrow recovery measured as cellularity, and hematopoietic colony-forming cells including primitive granulocyte-erythroid-megakaryocyte-monocytes (GEMM), reached pre-irradiation levels by day 30 in JP4-039 treated groups. Mice receiving single or multiple administrations of JP4-039 showed an early return of CFU-GEMM.
Conclusion
JP4-039 (GS-Nitroxide) is a safe radiation mitigator in mice warranting studies in larger animals and potentially a Phase I Clinical Trial.
Keywords: Hematopoietic syndrome, GS-nitroxide, radiation mitigation, total body irradiation
In complex mammalian organ systems, lethality following total-body irradiation (TBI) has been characterized into specific syndromes depending upon dose, dose rate, radiation quality including relative biologic effectiveness (RBE) of the energy of photon or particle beam irradiation, and histopathology of organs examined at time of death (1). The pathophysiology of radiation lethality in humans has been correlated with studies in rodents, dogs, and non-human primates, and follows specific dose- and time-related radiologic changes associated with lethality (2, 3). The hematopoietic syndrome (2–8 Gy total-body dose) in humans is associated with lethality at 30 days, and is defined by that radiation dose which can be rescued by hematopoietic stem cell transplantation (4). Within this dose range, there has been no consistent explanation as to why some individuals suffer radiation lethality at 2 Gy while others survive doses up to 8 Gy (4–6). Similarly, in animal model systems, examination of bone marrow cellularity, numbers of surviving hematopoietic stem cells, and numbers of committed hematopoietic multilineage progenitor cells, has shown no radiation dose-related significant differences at day 5 after TBI, in mice given the LD 10/30 (lethal dose for 10% of mice at 30 days) compared to the LD 70/30 dose (1). Furthermore, mice irradiated to the LD 70/30 TBI dose demonstrate survival of hematopoietic stem cells in the marrow that are capable of reconstituting transplant recipients (5). These data suggest that the cause of death following TBI may not be solely related to the destruction of hematopoietic stem cells.
Recent studies indicate that rodents surviving TBI suffer bradycardia and pericardial effusion, rather than tachycardia and high output failure, which would usually be associated with the anemia of hematopoietic failure (1). Other evidence also points to a critical role of intestinal flora in TBI-induced lethality in individuals suffering the hematopoietic syndrome (7–9).
The gastrointestinal (GI) syndrome is usually associated with higher TBI doses of 12–13 Gy in humans (10), and is defined as that dose which cannot be rescued by bone marrow transplantation (6, 11). Bone marrow transplantation-reduced lethality from intestinal radiation (12) may be in part attributable to the presence of progenitors of gastrointestinal epithelial and endothelial cells in the marrow transplant inoculum (13, 14). Direct irradiation-induced apoptosis of endothelial and intestinal epithelial cells has been demonstrated to be dose dependent and also cell phenotype-specific (14, 15).
To elucidate the role of each factor in TBI-induced death, and its modulation by new drugs, it is necessary to separate the role of each organ-specific variable (5) and to be careful that a new radiation mitigator not be toxic for animals likely to survive spontaneously. In the present studies, the effect of a new radiation mitigator, JP4-039 (17) was tested with respect to hematopoietic recovery. No toxicity of JP4-039 was found for low-dose-irradiated groups, but similarity in marrow responses between dose groups was also found, suggesting that other organ targets or variables, including the GI tract may determine survival from what has been termed the ‘hematopoietic syndrome’.
Materials and Methods
Animals and animal care
Female C57BL/6JHNsd adult mice weighing 30–33 g were housed five mice per cage and fed standard laboratory chow according to institutional The Institutional Animal Care and Use Committee (IACUC) protocols.
Total body irradiation
Mice were irradiated to 9.0, 9.15 or 9.25 Gy using a Gamma Cell Cesium Irradiator (JL Shepherd, San Fernando, CA, USA) (dose rate 70 cGy/min) according to previously published methods (16). All mice were un-anesthetized at the time of irradiation. Following irradiation, mice were returned to their original cages and monitored by institutional animal care technicians. Animals with body weight loss of 30% or more, or visible signs of dehydration or morbid behavior were sacrificed according to institutional IACUC protocols.
GS-nitroxide JP4-039 synthesis/formulation
Synthesis of JP4-039, a peptide isostere linked 4-amino-Tempo nitroxide, was performed as described previously (17). Drug was expended in F14 emulsion for intraperitoneal administration. F14 is an emulsion developed as an in vivo vehicle for JP4-039, which was originally used topically to mitigate irradiation damage to the skin (2). This formulation consists of a mixture of 10% sesame oil (Sigma-Aldrich, St. Louis, MO, USA), 5% soy phosphatidyl choline (Avanti Polar Lipids, Alabaster, AL, USA) and 85% Dulbecco’s phospate-buffered saline (Lonza, Walkersville, MD, USA). During the preparation, the active ingredient (JP4-039) was mixed with the other components before making the emulsions. Sonication was performed for 1–2 hours using a continuous model at 17–20 W output with ice water cooling and a stream of nitrogen blowing on top.
Hematological evaluation
Peripheral blood was obtained from sacrificed mice via the tail vein or by cardiac puncture. Blood, bone marrow (BM) and femur samples were taken from the same mouse for analysis at each time-point.
Complete blood counts
For complete blood counts (CBC), blood was collected in EDTA-treated MiniCollect tubes (Greiner-bio-one, Germany). Samples were analyzed using a scil Vet ABC hematology analyzer (scil Animal Care Company, Gurnee, IL, USA). Each sample was analyzed for the following: hemoglobin and hematocrit values, total white blood cell count (WBC), total red blood cell count (RBC), and lymphocyte, granulocyte, monocyte, neutrophil and platelet counts. Results are reported as the mean±standard deviation (results are presented as relative number given the absolute metrics for each parameter). The two-sided two-sample Student’s t-test was used to compare treatment groups.
Analysis of serum chemistries, liver and renal function tests
For serum chemistry, blood was collected in additive-free Microtainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA). Samples were centrifuged, serum removed and shipped overnight to Antech Diagnostics GLP laboratory (Morrisville, NC, USA) for analysis. Serum samples were analyzed for sodium, potassium, calcium, chloride, glucose, creatinine, albumin, total protein, and for the liver function markers AST and ALT. Results are reported as mean±standard deviation (tables) and mean±standard error of the mean (graphs).
Histopathologic evaluation of marrow cellularity
Light microscopic assessment of bone marrow cellularity was performed on decalcified formalin-fixed, hematoxylin and eosin (H&E)-stained paraffin sections of mouse femur. Sagittal sections of femur from sacrificed animals were prepared for histopathological analysis according to laboratory protocols. For each animal, two slides, with two fields/slide, were scored by three observers using a multi-head microscope, for a total of four fields/animal. Each observer scored the percent of marrow space with non-retraction of tissue, occupied by hematopoietic or stromal cells.
Results are reported as mean±standard deviation. The two-sided two-sample Student’s t-test was used to compare treatment groups. Hematopoietic cell colony forming assays. For clonogenic assays, tibial bone marrow cells were plated in triplicate in methylcellulose medium supplemented with rm SCF, rm IL-3, rm IL-6 and rm EPO (Stem Cell Technologies, Inc., Vancouver, BC, USA) for assessment of hematopoietic cells within the marrow capable of forming colonies in semi-solid medium in vitro. Colonies were scored on day 11 for colony-forming unit-granulocyte macrophage (CFU-GM), burst-forming unit erythroid (BFU-E), and colony forming unit – granulocyte-erythroid-megakaryocyte-monocyte (CFU-GEMM). Results are reported as the mean±standard deviation. The two-sided two-sample Student’s t-test was used to compare treatment groups.
Statistical analysis
All values were expressed as mean±standard deviation. The main period in vivo data was analyzed by ANOVA and statistical significance then tested by using the Student-Newman-Keuls test. Data sets having p<0.05 were considered significant. Experimental group size of n=15 were used for all in vivo experiments except where otherwise indicated. All in vitro experiments were carried out in individual mice and then mice within a group pool evaluation of statistics by Student’s t-test as previously published.
Results
JP4-039 is a safe and non-toxic radiation mitigator against the hematopoietic syndrome
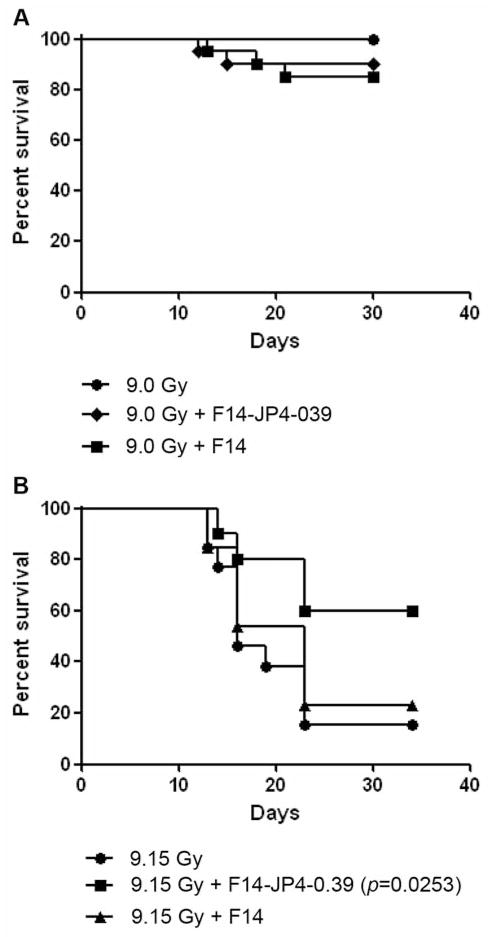
Groups of mice received intraperitoneal injection of JP4-039 at 10 mg/kg delivered 24 hours after irradiation. This time point was chosen to represent the time likely for human victims of radiation terrorism to reach a facility where a radiation mitigator could be administered. The results of these studies demonstrated a mitigative effect of 10 mg/kg against 9.15 but not 9.0 Gy at this time point (Figure 1). Mice receiving 15 or 20 mg/kg showed no further significant radiation mitigation (data not shown).
Figure 1.
Effect of JP4-039 (10 mg/kg in F14 formulation) on mouse survival after 9.0 or 9.15 Gy (n=5 for each group).
Due to the low toxicity of JP4-039 and the F14 formulation, the effect of JP4-039 delivered in F14 with respect to source parameters of hematopoietic recovery after 9.0 Gy was evaluated. Based on those results, the same parameters of hematopoietic recovery after 9.15 or 9.25 Gy TBI were then investigated. The results for the hematologic parameters (CBC, colony counts, and BM cellularity) are shown Tables I–VII. Representative photos of marrow are shown in Figure 2.
Table I.
Summary and analysis of the hematology data for each irradiated group at day 5.
| Group | WBC | RBC | Lymphocytes | Monocytes | Granulocytes | Neutrophils | Platelets | HGB | HCT |
|---|---|---|---|---|---|---|---|---|---|
| Non-irradiated control (n=9) | 2.0±2.2 | 5.0±2.5 | 1.3±1.7 | 0.0±0.1 | 0.5±0.5 | 371.9±384.3 | 276.3±261.7 | 9.0±4.1 | 25.5±13.1 |
| 9.0 Gy (n=5) | 0±0 P1=0.001 |
6.7±1.8 | 0±0 P1=0.006 |
0±0 | 0±0 P1=0.008 |
0±0 P1=0.002 |
356±381.3 | 10.0±2.3 | 33.2±8.9 |
| 9.0 Gy + F14 (n=5) | 1.1±0.8 | 7.5±0.8 P1=0.044 |
0.5±0.4 | 0.02±0.04 | 0.4±0.4 | 216.0±236.1 | 452.0±242.3 | 10.9±1.2 | 38.3±3.4 P1=0.039 |
| 9.15 Gy + F14 + JP4-039 (n=5) | 0.5±0.2 P1=0.014 |
7.1±1.7 | 0±0 P1=0.006 |
0±0 (n=5) | 0±0 P1=0.008 |
0±0 P1=0.002 |
484±376.0 | 9.7±3.2 | 35.3±8.6 |
| 9.15 Gy (n=7) | 0.7±0.5 P1=0.011 |
6.3±3.2 | 0.3±0.5 P1=0.011 |
0.0±0.0 P1=0.047 |
0.1±0.2 P1=0.0060 |
71.6±127.9 P1=0.0021 |
651.3±398.1 P1=0.049 |
9.4±4.9 | 32.4±16.7 |
| 9.15 Gy + F14 (n=7) | 0.6±0.3 P1=0.0071 |
7.6±2.6 | 0.0±0.0 P1=0.0018 |
0.0±0.0 P1=0.047 |
0.0±0.0 P1=0.0004 |
0.0±0.0 P1=0.0002 |
430.0±323.9 | 10.5±4.4 | 39.4±13.5 |
| 9.15 Gy + F14 + JP4-039 (n=7) | 0.9±0.4 P1=0.028 |
7.8±1.9 P1=0.044 |
0.2±0.4 P1=0.0087 |
0.0±0.0 P1=0.047 |
0.2±0.3 P1=0.033 |
126.9±177 P1=0.011 |
316.2±265.6 | 12.0±2.5 | 41.0±9.9 P1=0.033 |
| 9.25 Gy (n=7) | 0.3±0.4 P1=0.0016 |
8.9±1.6 P1=0.0069 |
0.0±0.0 P1=0.0018 |
0.0±0.0 P1=0.047 |
0.0±0.0 P1=0.0004 |
0.0±0.0 P1=0.0002 |
458.7±275.8 | 13.2±2.2 P1=0.042 |
46.2±8.8 P1=0.0053 |
| 9.25 Gy + F14 (n=7) | 0.0±0.0 P1=0.0002 |
5.5±3.9 P2=0.024 |
0.0±0.0 P1=0.0018 |
0.0±0.0 P1=0.047 |
0.0±0.0 P1=0.0004 |
0.0±0.0 P1=0.0002 |
418.7±417.1 | 8.1±5.5 P2=0.023 |
28.2±20.4 P2=0.020 |
| 9.25 Gy + F14 + JP4-039 (n=7) | 0.2±0.2 P1=0.0007 |
6.3±2.4 | 0.0±0.0 P1=0.0018 |
0.0±0.0 P1=0.047 |
0.0±0.0 P1=0.0004 |
0.0±0.0 P1=0.0002 |
587.4±546.0 | 9.6±3.4 | 31.9±12.6 |
N: Corresponding sample size; P1: p-value for the comparison with the non-irradiated control group; P2: p-value for the comparison between a treated group and the radiation-only group. Only significant p-values are reported.
Table VII.
JP4-039 Amelioration of the effect of 9. 0, 9.15 or 9.25 Gy TBI on marrow cellularity over 20 days.
| Group | Cellularity
|
|||
|---|---|---|---|---|
| Day 5 | Day 10 | Day 20 | Day 30 | |
| Non-irradiated Control n=9 | 93.5±2.5 | 93.5±2.5 | 93.5±2.5 | 93.5±2.5 |
| 9.0 Gy n=5 | 4.8+2.1 P1<0.0001 |
94.8±0.4 | ||
| 9.0 Gy +F14 n=5 | 3.7±1.4 P1<0.0001 |
95.0±0 | ||
| 9.0 Gy +F14 + JP4-039 n=5 | 3.8±2.9 P1<0.0001 |
96.0±0 | ||
| 9.15 Gy n=7 | 2.0±1.1 P1<0.0001 |
4.1±3.2 P1<0.0001 |
32.8±23.2 P1=0.0002 |
|
| 9.15 Gy + F14 n=7 | 1.9±1.1 P1<0.0001 |
22.7±31.8 P1<0.0001 P2=0.011 |
53.8±33.6 P1=0.012 |
|
| 9.15 Gy + F14 + JP4-039 | 2.2±0.8 P1<0.0001 n=7 |
14.3±9.4 P1<0.0001 n=6 |
60.6±37.3 P1=0.035 n=7 |
|
| 9.25 Gy | 1.7±0.6 P1<0.0001 n=7 |
4.7±1.9 P1<0.0001 n=6 |
39.3±38.1 P1=0.0013 n=6 |
|
| 9.25 Gy + F14 n=7 | 1.6±1.0 P1<0.0001 |
5.0±3.0 P1<0.0001 |
54.5±34.5 P1+0.013 |
|
| 9.25 Gy + F14 + JP4-039 | 1.0±0.1 P1<0.0001 n=7 |
4.2±2.6 P1<0.0001 n=5 |
42.9±30.7 P1=0.0026 n=6 |
|
Mean±standard deviation of cellularity in each group, where n is the corresponding sample size. The two-sided two sample t-test was used to compare between the non-irradiated control group and the other groups, between the radiation only group and the other groups and between each treated group and its vehicle control group. t-Tests showed that all the irradiated groups had significantly lower percentage cellularity than the non-irradiated group (all these p-values are <0.0001). All the other tests had non-significant p-values. P1 is the p-value for t-test comparing this group with the non-irradiated control group; P2 is the p-value for t-test comparing this treated group with the corresponding radiation-only group; P3 is the p-value for t-test comparing this group with the corresponding vehicle control (i.e. radiation + F14). Only significant p-values are shown.
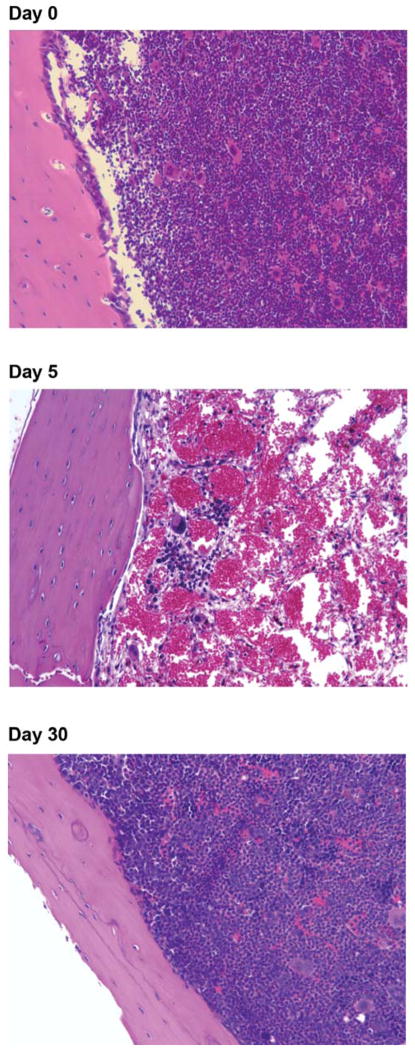
Figure 2.
H&E-stained bone sections showing the effect of 9.0 Gy TBI on marrow cellularity.
Effect of JP4-039 on radiation mitigation
JP4-039 delivered at 10 mg/kg in C57BL/6HNsd mice was evaluated with respect to improvement in survival after 9.0 Gy. Mice received either 9.0 Gy TBI only, 9.0 Gy+JP4-039 in F14 emulsion or 9.0 Gy+F14 alone. As shown in Figure 1A, 19 out of 20 mice in the JP4-039 treated group were alive at 30 days post 9.0 Gy. In the group of mice that received F14 only, 18 out of 20 mice were alive at 30 days post 9.0 Gy. This group of mice was then followed for evidence of hematopoietic recovery for 30 days. No statistically significant differences between groups were seen. The effect of JP4-039 after 9.15 Gy was investigated based on the survival of mice following 9.0 Gy and the fact that JP4-039 did not adversely affect survival. Administering JP4-039 after 9.15 Gy TBI was significantly mitigative, with 6 out of 10 mice surviving at 30 days, p=0.0253, (Figure 1B), compared to 3 out of 13 mice that received F14 only, and 2 out of 13 for the group that received irradiation only. These data provide evidence that JP4-039 administration after irradiation was mitigative and that F14 emulsion was a safe vehicle by which to deliver JP4-039.
The results demonstrate the importance of ensuring that a radiation mitigator will have minimal toxicity so that animals destined to recover from a relatively low dose of TBI (9.0 Gy) will not be adversely affected by its administration.
JP4-039 effective mitigation of the hematopoietic syndrome correlates with several measures of peripheral blood and bone marrow cellularity
The effect of JP4-039 on peripheral blood counts at day 5, day 10, and day 20 and 30 (9.0 Gy only) is shown in Tables I–III. By day 5 (Table I) WBC, lymphocyte, monocyte, granulocyte and neutrophil counts were consistently less than those of the non-irradiated control group. There was not a significant decrease in RBC, hemoglobin or hematocrit over the non-irradiated control group for all treatment groups. By day 10 (Table II), an increase in peripheral blood counts was evident. WBC counts decreased on day 5, but showed increased counts by day 10 and by day 20, exceeded the control in the 9.0, 9.15 and 9.25 Gy group that received JP4-039. This pattern was also seen with the lymphocyte, monocyte, granulocyte, and neutrophil counts. The RBC counts, platelet counts and the hemoglobin and hematocrit values remained consistent for all treatment groups at each time-point. There was no observed difference in the relative lethality of each irradiation dose reflected in each parameter peripheral blood counts.
Table III.
Summary and analysis of the hematology data for each irradiated group at day 20.
| Group | WBC | RBC | Lymphocytes | Monocytes | Granulocytes | Neutrophils | Platelets | HGB | HCT |
|---|---|---|---|---|---|---|---|---|---|
| Non-irradiated control (n=9) | 2.0±2.2 | 5.0±2.5 | 1.3±1.7 | 0.0±0.1 | 0.5±0.5 | 371.9±384.3 | 276.3±261.7 | 9.0±4.1 | 25.5±13.1 |
| 9.0 Gy (day 30) (n=5) | 3.8±2.2 | 6.4±2.1 | 2.0±1.0 | 0.2±0.2 P1=0.011 |
1.5±1.1 P1=0.013 |
1221.0±866.9 P1=0.013 |
382.0±218.3 | 9.9±3.1 | 33.3±11.1 |
| 9.0 Gy +F14 (day 30) (n=5) | 4.2±0.8 P1=0.042 |
6.4±1.9 | 2.4±0.3 | 0.2±0.1 P1=0.011 |
1.5±0.5 P1=0.014 |
1201.6±449.5 P1=0.015 |
460.8±142.1 | 10.2±2.5 | 34.0±9.6 |
| 9.0 Gy +F14 + JP4-039 (day 30) (n=5) | 4.1±1.8 P1=0.050 |
7.0±1.7 | 2.2±0.8 | 0.3±0.1 P1=0.005 |
1.6±0.9 P1=0.010 |
1165.0±632 P1=0.019 |
436.0±304.1 | 11.1±2.6 | 36.4±9.2 (n=5) |
| 9.15 Gy (n=7) | 4.4±8.4 | 2.0±0.8 P1=0.0008 |
2.8±5.7 | 0.2±0.4 | 1.2±2.4 | 840.4±1681.7 | 102.0±75.5 | 3.5±1.9 P1=0.0006 |
9.9±4.2 P1=0.0025 |
| 9.15 Gy + F14 (n=7) | 0.0±0.0 P2=0.022 |
4.0±2.4 P2=0.031 |
0.0±0.0 P2=0.031 |
0.0±0.0 | 0.0±0.0 | 0.0±0.0 P2=0.046 |
267.9±331.9 | 6.4±4.6 | 22.7±15.2 P2=0.017 |
| 9.15 Gy + F14 + JP4-039 (n=7) | 0.3±0.7 P2=0.042 |
2.3±0.8 P1=0.0016 |
0.0±0.0 P2=0.031 |
0.0±0.0 | 0.0±0.0 | 0.0±0.0 P2=0.046 |
160.9±82.6 P1=0.0005 |
3.3±1.6 | 11.5±4.2 P1=0.0060 P3=0.034 |
| 9.25 Gy (n=7) | 0.5±0.7 | 2.4±0.3 P1=0.0024 |
0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 104.9±39.2 | 3.5±0.4 P1=0.0008 |
10.8±1.2 P1=0.0042 |
| 9.25 Gy + F14 (n=7) | 2.8±1.8 | 3.0±1.2 P1=0.018 |
1.2±1.2 | 0.1±0.1 | 1.2±1.8 | 733.2±908.8 | 138.4±105.3 | 4.9±2.1 P1=0.010 |
16.8±8.1 |
| 9.25 Gy + F14 + JP4-039 | 3.1±2.8 | 3.1±1.6 P1=0.023 |
2.1±1.9 | 0.1±0.2 | 0.8±0.8 | 673.9±673.7 | 138.9±111.0 | 4.8±3.1 P1=0.0084 |
17.2±10.8 |
N: Corresponding sample size; P1: p-value for the comparison with the non-irradiated control group; P2: p-value for the comparison between a treated group and the radiation-only group; P3: p-value for the comparison between a treated group and its corresponding vehicle control. Only significant p-values are reported.
Table II.
Summary and analysis of the hematology data for each irradiated group at day 10.
| Group | WBC | RBC | Lymphocytes | Monocytes | Granulocytes | Neutrophils | Platelets | HGB | HCT |
|---|---|---|---|---|---|---|---|---|---|
| Non-irradiated control (n=9) | 2.0±2.2 | 5.0±2.5 | 1.3±1.7 | 0.0±0.1 | 0.5±0.5 | 371.9±384.3 | 276.3±261.7 | 9.0±4.1 | 25.5±13.1 |
| 9.15 Gy (n=7) | 1.1±0.9 | 5.8±0.8 | 0.5±0.9 | 0.0±0.0 | 0.3±0.3 | 192.1±221.6 (n=6) | 65.4±34.6 P1=0.0017 |
8.8±1.3 | 28.4±4.0 |
| 9.15 Gy + F14 (n=7) | 2.6±4.4 | 5.8±1.6 | 1.8±3.7 | 0.1±0.2 | 0.6±0.7 | 238.7±194.4 | 119.7±65.5 P1=0.017 |
9.0±2.3 | 24.5±12.5 |
| 9.15 Gy + F14 + JP4-039 (n=5) | 1.2±0.8 | 5.0±1.1 | 0.3±0.6 | 0.0±0.0 | 0.2±0.5 | 109.0±243.7 | 70.4±65.4 P1=0.0051 |
7.4±1.6 | 24.1±5.5 |
| 9.25 Gy (n=6) | 1.3±0.7 | 6.3±1.2 | 0.5±0.6 | 0.0±0.0 | 0.4±0.4 | 192.9±226.5 | 90.2±57.6 P1=0.0072 |
9.4±1.9 | 30.8±5.8 |
| 9.25 Gy + F14 (n=7) | 0.9±0.5 | 4.9±2.0 | 0.3±0.5 | 0.0±0.0 | 0.1±0.3 | 703.8±1820.7 | 71.7±27.0 P1=0.0023 |
7.2±3.2 | 24.4±10.2 |
| 9.25 Gy + F14 + JP4-039 (n=6) | 0.8±0.3 | 5.6±0.9 | 0.1±0.2 | 0.0±0.0 | 0.1±0.2 | 56.2±137.7 | 65.5±23.9 P1=0.0026 |
8.1±1.1 | 22.1±8.7 |
N: Corresponding sample size; P1: p-value for the comparison with the non-irradiated control group. Only significant p-values are reported.
Tables IV–VI show the effect of JP4-039 on colony forming cell recovery at day 5, day 10, day 20 and day 30 (9.0 Gy only) of specific colony subtypes (CFU-GM, BFU-E, CFU-GEMM) and total colony numbers. At day 5 (Table IV) and day 10 (Table V) all colony counts were significantly less than the non-irradiated control group. Colony counts had begun to recover by day 20, but remained lower than non-irradiated controls (Table VI). CFU-GEMM recovered earlier in JP4-039 treated groups (Table VI).
Table IV.
Effect of JP4-039 on irradiation response of marrow colony forming cells at day 5.
| Group | CFU-GM | BFU-E | CFU-GEMM | Total |
|---|---|---|---|---|
| Non-irradiated control (n=9) | 373.1±42.7 | 66.7±14.2 (n=9) | 15.6±5.7 | 455.4±46.8 |
| 9.0 Gy (n=5) | 12.4±12.5 | 0.8±1.2 | 0±0 | 1.32±13.7 |
| 9.0 Gy +F14 (n=5) | 10.4±7.5 | 0.1±0.3 | 0±0 | 10.5±13.7 |
| 9.0 Gy +F14 + JP4-039 (n=5) | 28.0±11.5 | 0.6±0.8 | 0±0 | 28.6±11.9 |
| 9.15 Gy (n=7) | 18.4±12.0 P1<0.0001 |
4.8±4.2 P1<0.0001 |
0.4±0.5 P1<0.0001 |
23.6±16.0 P1<0.0001 |
| 9.15 Gy + F14 (n=7) | 16.4±8.1 P1<0.0001 |
4.2±2.8 P1<0.0001 |
0.5±0.4 P1<0.0001 |
21.1±10.6 P1<0.0001 |
| 9.15 Gy + F14 + JP4-039 (n=7) | 24.4±13.1 (n=7) P1<0.0001 |
2.5±1.8 P1<0.0001 |
0.5±0.7 P1<0.0001 |
27.4±14.1 P1<0.0001 |
| 9.25 Gy (n=7) | 15.9±8.8 P1<0.0001 |
2.8±1.1 P1<0.0001 |
0.0±0.0 P1<0.0001 |
18.6±8.4 P1<0.0001 |
| 9.25 Gy + F14 (n=7) | 12.0±4.3 P1<0.0001 |
3.7±3.2 P1<0.0001 |
0.1±0.3 P1<0.0001 |
15.7±7.1 P1<0.0001 |
| 9.25 Gy + F14 + JP4-039 (n=7) | 21.7±11.0 (n=7) P1<0.0001 |
5.3±2.7 P1<0.0001 |
0.2±0.3 P1<0.0001 |
27.2±12.9 P1<0.0001 |
Mean±standard deviation of average number of colonies per 105 cells plated, where n is the corresponding sample size. P1: p-value for t-test comparing this group with the non-irradiated control group; all P1 are <0.0001. Only significant p-values are shown.
Table VI.
Effect of JP4-039 on irradiation response of marrow colony forming cells at day 20.
| Group | CFU-GM | BFU-E | CFU-GEMM | Total |
|---|---|---|---|---|
| Non-irradiated control (n=9) | 373.1±42.7 | 66.7±14.2 (n=9) | 15.6±5.7 | 455.4±46.8 |
| 9.0 Gy (day 30) (n=5) | 202.3±48.0 P1<0.0001 |
37.7±7.0 P1=0.013 |
3.0±2.2 P1<0.0001 |
243.0±46.8 P1<0.0001 |
| 9.0 Gy +F14 (day 30) (n=5) | 210.7±77.4 P1<0.0001 |
68.0±30.1 P2=0.021 |
2.0±2.2 P1<0.0001 |
280.7±105.5 P1<0.0001 |
| 9.0 Gy +F14 + JP4-039 (day 30) (n=5) | 222.0±61.3 P1<0.0001 |
68.7±3.0 P2=0.019 |
3.3±1.2 P1<0.0001 |
294.0±63.7 P1=0.0002 |
| 9.15 Gy (n=7) | 34.6±27.1 P1<0.0001 |
8.2±5.8 P1<0.0001 |
0.6±0.8 P1<0.0001 |
43.3±32.9 P1<0.0001 |
| 9.15 Gy + F14 (n=7) | 165.7±134.9 P1<0.0001 |
40.7±37.2 P1<0.0001 P2=0.0077 |
0.5±1.3 P1<0.0001 |
206.9±172.2 P1<0.0001 P2=0.0030 |
| 9.15 Gy + F14 + JP4-039 (n=7) | 48.2±40.2 P1<0.0001 P3=0.0067 |
10.3±6.0 P1<0.0001 P3=0.012 |
0.5±0.3 P1<0.0001 |
59.0±45.7 P1<0.0001 P3=0.0068 |
| 9.25 Gy (n=7) | 113.1±107.9 P1<0.0001 |
25.2±24.8 P1<0.0001 |
0.2±0.6 P1<0.0001 |
138.6±132.2 (n=7) P1<0.0001 |
| 9.25 Gy + F14 (n=7) | 100.7±51.6 P1<0.0001 |
35.2±21.8 P1<0.0001 |
0.5±0.8 P1<0.0001 |
136.4±71.9 P1<0.0001 |
| 9.25 Gy + F14 + JP4-039 (n=7) | 122.4±80.1 P1<0.0001 |
45.2±26.0 P1<0.0001 |
2.4±2.7 P1<0.0001 |
170.0±105.5 P1<0.0001 |
Mean±standard deviation of average number of colonies per 105 cells plated, where n is the corresponding sample size. P1: p-value for t-test comparing this group with the non-irradiated control group; P2: p-value for t-test comparing this treated group with the radiation-only group; P3: p-value for t-test comparing this group with the corresponding vehicle control; all P1 are <0.0001. Only significant p-values are shown.
Table V.
Effect of JP4-039 on irradiation response of marrow colony forming cells at day 10.
| Group | CFU-GM | BFU-E | CFU-GEMM | Total |
|---|---|---|---|---|
| Non-irradiated control (n=9) | 373.1±42.7 | 66.7±14.2 (n=9) | 15.6±5.7 | 455.4±46.8 |
| 9.15 Gy (n=7) | 48.9±82.6 P1<0.0001 |
9.1±15.1 P1<0.0001 |
0.5±0.9 P1<0.0001 |
58.5±98.6 P1<0.0001 |
| 9.15 Gy + F14 (n=7) | 37.6±32.7 P1<0.0001 |
12.3±12.5 P1<0.0001 |
0.5±0.6 P1<0.0001 |
50.4±44.9 P1<0.0001 |
| 9.15 Gy + F14 + JP4-039 (n=6) | 8.3±11.8 P1<0.0001 |
1.5±2.3 P1<0.0001 |
0.1±0.2 P1<0.0001 |
9.9±14.1 P1<0.0001 |
| 9.25 Gy (n=6) | 34.1±59.2 (n=6) P1<0.0001 |
3.1±4.4 P1<0.0001 |
0.3±0.4 P1<0.0001 |
37.5±62.7 P1<0.0001 |
| 9.25 Gy + F14 (n=7) | 6.5±10.2 P1<0.0001 |
1.0±2.0 P1<0.0001 |
0.1±0.3 P1<0.0001 |
7.6±12.0 P1<0.0001 |
| 9.25 Gy + F14 + JP4-039 (n=4) | 22.6±28.0 P1<0.0001 |
1.8±2.1 P1<0.0001 |
0.7±0.9 P1<0.0001 |
25.0±30.3 P1<0.0001 |
Mean ± standard deviation of average number of colonies per 105 cells plated, where n is the corresponding sample size. P1: p-value for t-test comparing this group with the non-irradiated control group; P2: p-value for t-test comparing this treated group with the radiation-only group; P3: p-value for t-test comparing this group with the corresponding vehicle control; all P1 are <0.0001. Only significant p-values are shown.
Since the hemogram and marrow colony count data were not able to distinguish 9.0 and 9.15 Gy-treated from 9.25 Gy-treated animals, H&E-stained femur sections from the same mice used for the CBC and colony forming assays were next examined. The bone marrow cellularity at day 5 after irradiation was markedly reduced in 9.0 Gy irradiated controls as well as JP4-039 treated groups (Figure 2 and Table VII) (no difference between groups detected). There was bone marrow recovery by day 10, additional recovery by day 20 and full recovery by day 30 (Figure 2). There was no detectable difference in the bone marrow cellularity between the two doses (9.15 Gy and 9.25 Gy) at each of the three time-points.
JP4-039 has no deleterious effect on serum electrolytes, or liver function tests, parameters of bone marrow cellularity, or blood cell production
Comparison of mice receiving JP4-039 24 hours after irradiation with control irradiated (Figure 1) revealed that the drug was non-toxic compared to F14 formulation alone. Serum chemistry panel for Na, Ca, Cl albumin, glucose, protein and the A/G ratio also showed lack of any detectable deleterious effect of JP4-039 on serum markers of liver function (data not shown). The beneficial effect of JP4-039 stimulating recovery of CFU-GEMM is shown in Table VI. These data demonstrate that with low TBI doses of 9.0 or 9.15 Gy, there was no detectable toxicity of JP4-039/F14.
The effect of administration of JP4-039 at 10 mg/kg was investigated on mice receiving 9.0, 9.15 or 9.25 Gy TBI. There was no significant increase in survival by F14 emulsion alone. Marrow cellularity, marrow cell colony forming capacity, as well as serum chemistries were investigated in order to define time-points, days 5 and 30, and also observe marrow cellularity in animals dying in between those times. There was a significant reduction in bone marrow histopathological cellularity, colony forming progenitor cells (Table IV), and peripheral blood counts at day 5 in all irradiated groups. It was not possible to use the decrease in cellularity at day 5 in order to distinguish between groups receiving 9.0, 9.15 or 9.25 Gy, indicating that this histopathological measure cannot be used to predict likelihood of survival.
At day 5 after irradiation, there was a significant reduction in peripheral blood lymphocyte and granulocyte counts. Colony forming numbers in bone marrow were significantly reduced in all JP4-039 treatment groups and irradiated control groups. In an evaluation of individual mice dying between days 5 and 30, there was an acceleration in the cellular proliferation, leading to return of bone marrow cellularity between days 14–20. By day 30, there was a complete return to 95% marrow space cellularity that was indistinguishable from JP4-039-treated or control-irradiated mice in all irradiated and JP4-039 treated groups. These results provide evidence that JP4-039 was not associated with a significant reduction in bone marrow cellularity or a delay in recovery of marrow cellularity compared to irradiated and vehicle treated controls or irradiation controls.
Discussion
TBI responses by homogeneous populations of inbred mice, as well as heterogeneous populations of outbred animals (including humans) can vary significantly despite a uniform radiation dose and dose rate. Variables that can affect irradiation dose response include the combined injury effects of psychological stress, heterogeneity in intestinal flora, and co-morbid injury. Variation in dose response to TBI, in an inbred genetically identical mouse population, assayed over several months or several years, has also been reported (4). Analysis of new radiation mitigators for potential identification of lead compounds must take into account the background TBI sensitivity and responsiveness of the animals tested.
Since dosimetry methods for determining actual irradiation exposure dose in humans often are limited and based on distance from the radiation source, there will be individuals receiving doses that are actually lower than those estimated. Therefore, it is important that any radiation mitigator that is designed to be administered after TBI is safe and non-toxic for those individuals destined to spontaneously recover from a given TBI dose. Such individuals should not be disadvantaged by receiving a mitigator that would be needed by an individual receiving a higher dose. Furthermore, since responses to a low dose of radiation will vary between individuals, a mitigator should not compromise the respiratory or intestinal barrier function during the time of pancytopenia.
The analysis of new potentially valuable radiation mitigator, JP4-039, was carried out using C57BL/6HN mice. A novel safe and non-toxic formulation designated F14 was designed to deliver JP4-039. The formulation by itself provided no toxicity and when drug was delivered in the formulation, there was a measurable radiation mitigating effect against 9.15 Gy TBI.
The present studies demonstrated the difficulty in using blood counts, bone marrow cellularity, and bone marrow colony forming numbers as a measure of not only radiation dose, but the likelihood of animal survival from irradiation. In each experiment, administration of JP4-039 did not further decrease the parameters of hematopoietic recovery from radiation, and animals receiving a single dose or multiple daily JP4-039 drug doses beginning at 24 hours after 9.0 Gy, showed increased recovery of multilineage CFU-GEMM.
The pathophysiology of death in TBI experimental animals is not well understood. Much attention has been directed towards the radiobiology of specific cell phenotypes within the intestine and bone marrow (14, 15). However, little attention has been directed toward the role of intestinal flora and, specifically, sub-types of bacterial pathogens, which can contribute to lethality in specific individuals within a uniformly irradiated population (7–9, 18). Supportive care regimens, including broad-spectrum antibiotics, have been shown to increase the LD 50/30 dose in both rodent and dog models, and a further increase in TBI radioresistance has been demonstrated following the addition of antifungal agents to the supportive care regimen (4, 19). A potentially complicating variable in all of these studies is the recent demonstration of the radiation-mitigative capacity of specific classes of antibiotics (3, 20). Perhaps the most complicating variable in analysis of the role of the intestine in what has been termed the hematopoietic syndrome is the simultaneous drop in peripheral blood neutrophils, monocytes, and lymphocytes from the direct irradiation effect at the time when the intestinal mucosal barrier breakdown would be expected to lead to both the elaboration of inflammatory cytokines and the introduction into the peripheral blood of intestinal infectious pathogens (7–9, 18).
The appropriate interventions in patients exposed to TBI from accidents or radiation terrorist events must take into account the real possibility that many individuals will recover with no need for pharmacologic intervention. While antibiotics and antifungal agents used in the supportive care of TBI clinical marrow transplant patients or animal models of TBI in marrow transplant are clearly advantageous, there may be no compelling reason to administer such agents to large numbers of radiation exposed humans when the irradiation dose sustained is not known and when potentially toxic effects of antimicrobials may be avoided. Finally, in patients sustaining combined injury (radiation plus burn, wound, chemical injury, infection), pharmacological intervention for the non-irradiation component of the injury may be necessary. In these situations, drugs should be chosen which have minimal toxicity with respect to recovery from irradiation. The present studies show that JP4-039 is non-toxic to mice exposed to sub-lethal doses of TBI and is a safe radiation mitigator against the hematopoietic syndrome. Further studies will be required to determine the safety of JP4-039 in combined injury models.
Acknowledgments
This work was supported by a grant from the NIAID/NIH 1U19A168021 and BAA-BARDA Contract HHS 0150250800002C.
References
- 1.Vriesendorp HM, van Bekkum DW. Susceptibility to total-body irradiation. In: Broerse JJ, MacVittie T, editors. Response to Total-Body Irradiation in Different Species. Amsterdam: Martinus Nijhoff; 1984. [Google Scholar]
- 2.Epperly M, Brand R, Stottlemyer J, Dixon TM, Gao X, Li S, Huq S, Wipf P, Falo LD, Greenberger JS. American Society for Therapeutic Radiology and Oncology, Abstract. San Diego, CA: 2010c. Topical application of GS-nitroxide JP4-039 emulsion mitigation ionizing irradiation induced skin burns. [Google Scholar]
- 3.Kim K, Pollard JM, Norris AJ, McDonald JT, Sun Y, Micewicz E, Pettijohn K, Damoiseaux R, Iwamoto KS, Sayre JW, Price BD, Gatti RA, McBride WH. High-throughput screening identifies two classes of antibiotics as radioprotectors: tetracyclines and fluoroquinolones. Clin Cancer Res. 2009;15(23):7238–7245. doi: 10.1158/1078-0432.CCR-09-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone HB, Coleman CN, Moulder JE, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, Hauer-Jensen M, Hill RP, Kolesnick RN, MacVittie TJ, Marks C, McBride WH, Metting N, Pellmar T, Purucker M, Robbins MEC, Schiestl RH, Seed TM, Tomaszewski J, Travis EL, Wallner PE, Wolpert M, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation, and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162:711–718. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 5.Vriesendorp HM, Chu H, Ochran TG, Besa PC, Champlin RE. Radiobiology of total body radiation. Bone Marrow Transplantation. 1994;14(Suppl):S4–S8. [PubMed] [Google Scholar]
- 6.Thomas ED. Observations on supralethal whole-body irradiation and marrow transplantation in man and dog. Ann NY Acad Sci. 1964;114:393–402. doi: 10.1111/j.1749-6632.1964.tb53592.x. [DOI] [PubMed] [Google Scholar]
- 7.Haralambie E, Schmidt-Weinmar A. Infections after experimental cadaver bone marrow transplantation in beagle dogs. Transplantations with and without selective gastrointestinal decontamination. Infection. 1988;16:36–41. doi: 10.1007/BF01646930. [DOI] [PubMed] [Google Scholar]
- 8.Vriesendorp HM, Heidt PJ, Zurcher C. Gastrointestinal decontamination of dogs treated with total body irradiation and bone marrow transplantation. Exp Hematol. 1981;9:904–916. [PubMed] [Google Scholar]
- 9.Puzova H, Szabova K, Cerman SJ, Puza A, Kunstadt E, Zaduban M. The problem of autoinfection after total-body lethal irradiation of dogs with 60Co. Folia Biol. 1962;8:298–308. [PubMed] [Google Scholar]
- 10.Hemplemann LH, Lisco H, Hoffman JD. The acute radiation syndrome: a study of nine cases and a review of the problem. Anna Intern Med. 1952;36:279–510. doi: 10.7326/0003-4819-36-2-279. [DOI] [PubMed] [Google Scholar]
- 11.Thomas ED, Storb R, Buckner CD. Total-body irradiation in preparation for marrow engraftment. Transplant Proc. 1976;8:591–593. [PubMed] [Google Scholar]
- 12.Travis EL, Peters LJ, McNeill J, Thames HD, Jr, Karolis C. Effect of dose-rate on total-body irradiation: lethality and pathologic findings. Radiother Onc. 1985;4:341–351. doi: 10.1016/s0167-8140(85)80122-5. [DOI] [PubMed] [Google Scholar]
- 13.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 14.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 15.Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, Zhang L, Yu J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epperly M, Jin SQ, Nie S, Cao S, Zhang X, Franicola D, Fink M, Greenberger JS. Ethyl pyruvate, a potentially effective total body irradiation damage mitigator. Radiat Res. 2007;168:552–559. doi: 10.1667/RR1009.1. [DOI] [PubMed] [Google Scholar]
- 17.Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, Zhao H, Tyurin VA, Pierce JG, Kagan VE, Kanai AJ, Greenberger JS. The mitochondrial targeted JP4-039 augments potentially lethal irradiation damage. In Vivo. 2009;23:717–726. [PMC free article] [PubMed] [Google Scholar]
- 18.Weisdorf D, Chao N, Waselenko JK, Dainiak N, Armitage JO, McNiece I, Confer D. Acute radiation injury: contingency planning for triage, supportive care, and transplantation. Biol Blood Marrow Transplant. 2006;12:672–682. doi: 10.1016/j.bbmt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Chao N. Hematopoietic stem cell transplantation. Curr Opin Hem. 2004;11:373–374. doi: 10.1097/01.moh.0000144500.82861.c0. [DOI] [PubMed] [Google Scholar]
- 20.Epperly MW, Franicola D, Shields D, Rwigema J-C, Stone B, Zhang X, McBride W, Georges G, Wipf P, Greenberger JS. Screening of antimicrobial agents for in vitro radiation protection and mitigation capacity, including those used in supportive care regimens for bone marrow transplant recipients. In Vivo. 2010a;24:9–20. [PMC free article] [PubMed] [Google Scholar]