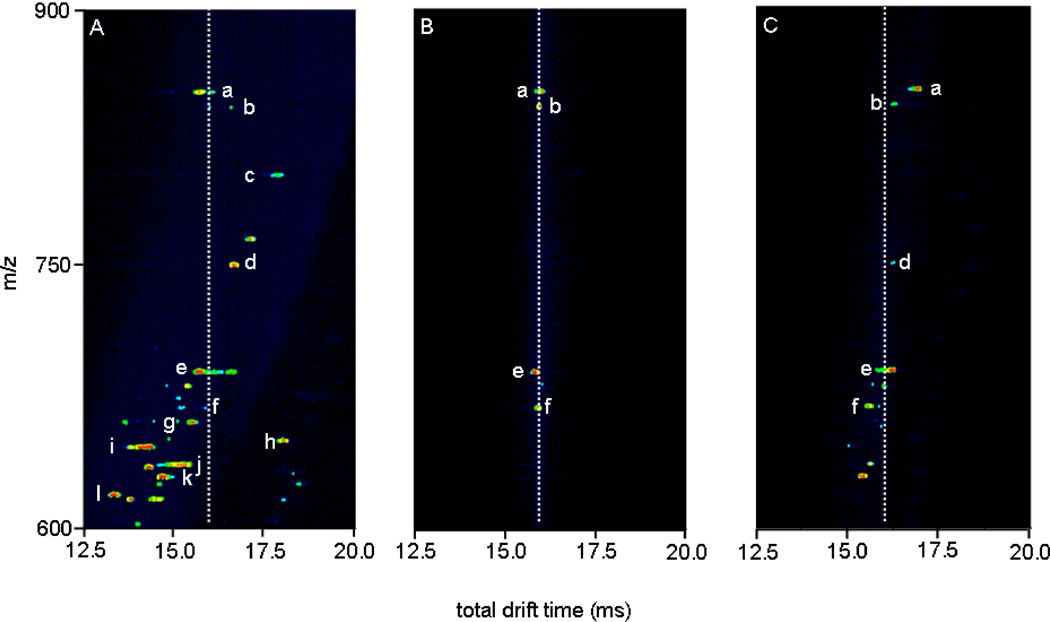
Figure 4.
Nested drift(flight) time distributions for horse myoglobin tryptic digest, total distribution (A, left), ions mobility selected at G2 (tD1 = 7.55–7.65 ms) with IA2 = 0 V (B, center), and same selection with IA2 = 120 V(C, right). The dotted lines denote the arrival times for inactivated mobility-selected ions, to show the degree of separation. a, [G1-R31+4H]4+; b, [Y103-K133+4H]4+; c, [K63-K78+2H]2+ or [H64-K79+2H]2+; d, [H119-K133+2H]2+; e, [H64-K67+2H]2+; f, [V68-K77+2H]2+; g, [K79-K96+3H]3+; h, [L32-K47+3H]3+; i, [L32-K42+2H]2+; j, [Y103-K118+3H]3+; k, [G80-K96+3H]3+; l, [E148-G153+H]+. Note that the nomenclature used refers to the position of the peptide by providing the location (with respect to the intact protein sequence) and single letter abbreviation of the N- and C-terminal residues, respectively.