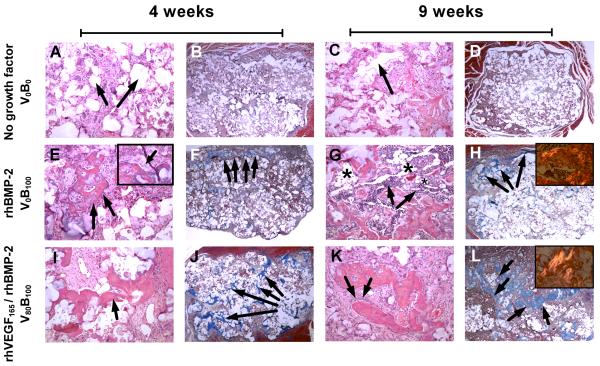
Fig. 7.
Histology sections from rat femur intramuscular implants. (A to D) Bone is absent in control scaffolds releasing no growth factors at 4 and 9 weeks verified by (A, C) H&E staining and (B, D) lack of brilliant blue collagen staining with Masson’s trichrome stain. (A, B) Arrows indicate pockets where the (PCL/βTCP) polymer scaffold is present at 4 weeks which degrades over (C, D) 9 weeks, without any bone growth in the vacant space. (E to H) Bone present in scaffolds releasing single growth factor rhBMP-2. (E) Presence of trabecular bone with (inset) osteoblasts laying down new bone which is (F) restricted to the periphery of the scaffold at 4 weeks. Of particular interest is (G) the abundant presence of hematopoietic cells surrounding the bone in spaces identified as fatty marrow space (asterisk), where (H) most of the bone formation is restricted to the periphery at 9 weeks and (inset) as bone matures, it goes from an unorganized collagen matrix structure to a lamellar structure with aligned collagen fibrils that are birefringent under polarized light. (I to L) Bone present in scaffolds releasing rhBMP-2 and rhVEGF165. (I) Spicules of trabecular bone which is present (J) throughout the scaffold as early as 4 weeks. (K) As osteoblasts lay down new bone, spicules mature into (L) bone that fills up the empty spaces throughout the scaffold as it degrades with (inset) a greater number of birefringent aligned collagen fibrils present than fibrils formed when single growth factor rhBMP-2 is released. Bone formed is always within the scaffold. A, C, E, G, I, K are hematoxylin and eosin (H&E) stains at 10× objective magnification. B, D, F, H, J, L are Masson’s trichrome stains at 2× objective magnification. Images in inset are taken at 20× objective magnification.