Abstract
BACKGROUND & AIMS
Helicobacter pylori-induced gastric carcinogenesis has been linked to the microbial oncoprotein CagA. Spermine oxidase (SMO) metabolizes the polyamine spermine into spermidine and generates H2O2 that causes apoptosis and DNA damage. We determined if pathogenic effects of CagA are attributable to SMO.
METHODS
Levels of SMO, apoptosis, and DNA damage (8-oxoguanosine) were measured in gastric epithelial cell lines infected with cagA+ or cagA− H. pylori strains, or transfected with a CagA expression plasmid, in the absence or presence of SMO small interfering RNA, or an SMO inhibitor. The role of CagA in induction of SMO and DNA damage was assessed in H. pylori-infected gastritis tissues from humans, gerbils, and both wild-type and hypergastrinemic INS-GAS mice, using immunohistochemistry and flow cytometry.
RESULTS
cagA+ strains or ectopic expression of CagA, but not cagA− strains, led to increased levels of SMO, apoptosis, and DNA damage in gastric epithelial cells, and knockdown or inhibition of SMO blocked apoptosis and DNA damage. There was increased SMO expression, apoptosis, and DNA damage in gastric tissues from humans infected with cagA+, but not cagA− strains. In gerbils and mice, DNA damage was CagA-dependent and present in cells that expressed SMO. Gastric epithelial cells with DNA damage that were negative for markers of apoptosis accounted for 42–69% of cells in gerbils and INS-GAS mice with dysplasia and carcinoma.
CONCLUSIONS
By inducing SMO, H. pylori CagA generates cells with oxidative DNA damage, and a subpopulation of these cells are resistant to apoptosis and thus at high risk for malignant transformation.
Keywords: polyamines, epithelial cells, DNA damage
Introduction
Infection with Helicobacter pylori is associated with an increased risk for gastric cancer1, the second leading cause of cancer death worldwide.2 H. pylori selectively colonizes the human gastric mucosa and infects half of the world’s population, including 30–40% in the United States.3 H. pylori interacts with gastric epithelial cells and cause activation of signaling cascades that result in dysregulation of diverse biological functions.3,4 Infection with H. pylori causes an increase in gastric epithelial apoptosis in vitro and in tissues from infected patients,5, 6 and this has been implicated in gastric carcinogenesis.3,4 However, H. pylori infection increases expression of pro- and anti-apoptotic proteins,6 and epidermal growth factor receptor activation by H. pylori limits the amount of gastric epithelial cell apoptosis in cell lines and infected mice.7 We have demonstrated that polyamines mediate H. pylori-induced apoptosis, and this occurs by upregulation of spermine oxidase (SMO), which back-converts the polyamine spermine to spermidine, and produces H2O2 in this process that causes mitochondrial membrane depolarization and caspase activation leading to apoptosis.8, 9
Oxidative stress that occurs during interactions between H. pylori and gastric epithelial cells can lead to DNA damage in vivo in humans and mice by causing nucleotide oxidation.10, 11 Chronic H. pylori infection increases mutation frequency leading to base substitutions and frame shift mutations12 and accumulation of cells with damaged DNA may occur by downregulation of pro-apoptotic proteins.13 These findings highlight the importance of oxidative stress during H. pylori infection, as the fate of cells with damaged DNA is critical in the development of H. pylori-induced cancer.
The cag pathogenicity island of H. pylori has been implicated in disruption of gastric epithelial cell function and morphology.3, 4, 14 The cag island comprises 31 putative genes, including cagA, encoding an effector molecule (CagA), and others, including cagE, that are required for assembly of a type IV secretion system that delivers CagA into gastric epithelial cells. 3,4, 15 Subjects infected with cagA+ H. pylori strains exhibit higher levels of gastric mucosal inflammation, and increased risk of development of premalignant atrophic gastritis and intestinal metaplasia.16 CagA seropositivity is associated with an increased risk of gastric cancer compared to cagA− infection,17,18 and advanced tumor stage.16, 18 Moreover, CagA overexpressing transgenic mice develop gastric adenocarcinomas.19 The biochemical and cellular mechanisms responsible for the association between CagA and gastric carcinogenesis have not been defined. We now demonstrate that H. pylori CagA induces DNA damage and apoptosis in gastric epithelial cells in vitro and in vivo in a SMO-dependent manner. In addition, in the gerbil and Insulin-Gastrin (INS-GAS) models of gastric cancer, a substantial percentage of SMO-expressing cells with DNA damage are resistant to apoptosis. Thus, we have identified a molecular mechanism for CagA-associated gastric carcinogenesis.
Materials and Methods
Reagents
Bacteria
The human H. pylori gastric ulcer strain B128,20 its gerbil-adapted oncogenic derivative 7.13,20 isogenic mutants of 7.13,20 as well as strain 60190 and its cagA isogenic mutant21 were used. Strain PMSS1 and its cagE isogenic mutant was provided by M. Amieva.22
Cell and Culture Conditions
Mouse conditionally-immortalized stomach epithelial (ImSt) cells7, 23 and human gastric cancer AGS cells were grown and co-cultured with H. pylori.7, 9
Animal Infections
C57BL/6 mice were infected with wild-type and cagE− PMSS1,22 and gerbils were infected with 7.13 or its isogenic cagA mutant.24 Insulin-Gastrin (INS-GAS) mice were infected with PMSS1.
Human Subjects
Gastric tissues were used as described,20 and as detailed in Supplementary Methods.
Additional Molecular Assays
Transient Transfection of cagA
The plasmid pSP65SRα containing cagA was transfected in ImSt cells; see Supplementary Methods.
Immunohistochemistry for SMO and 8-hydroxy-2'-deoxyguanosine (8-OHdG)
Detection of Apoptosis in Gastric Tissues
Apoptotic cells were identified by in situ oligo ligation (ISOL).7
Isolation of Epithelial Cells from Gastric Tissue
Gastric epithelial cells were isolated from frozen samples by dissociation and dispersion.23
Statistical Analysis
Quantitative data are shown as the mean ± SE. Analyses are in the Supplementary Methods.
Results
Spermine Oxidase Induction by H. pylori Is CagA-Dependent
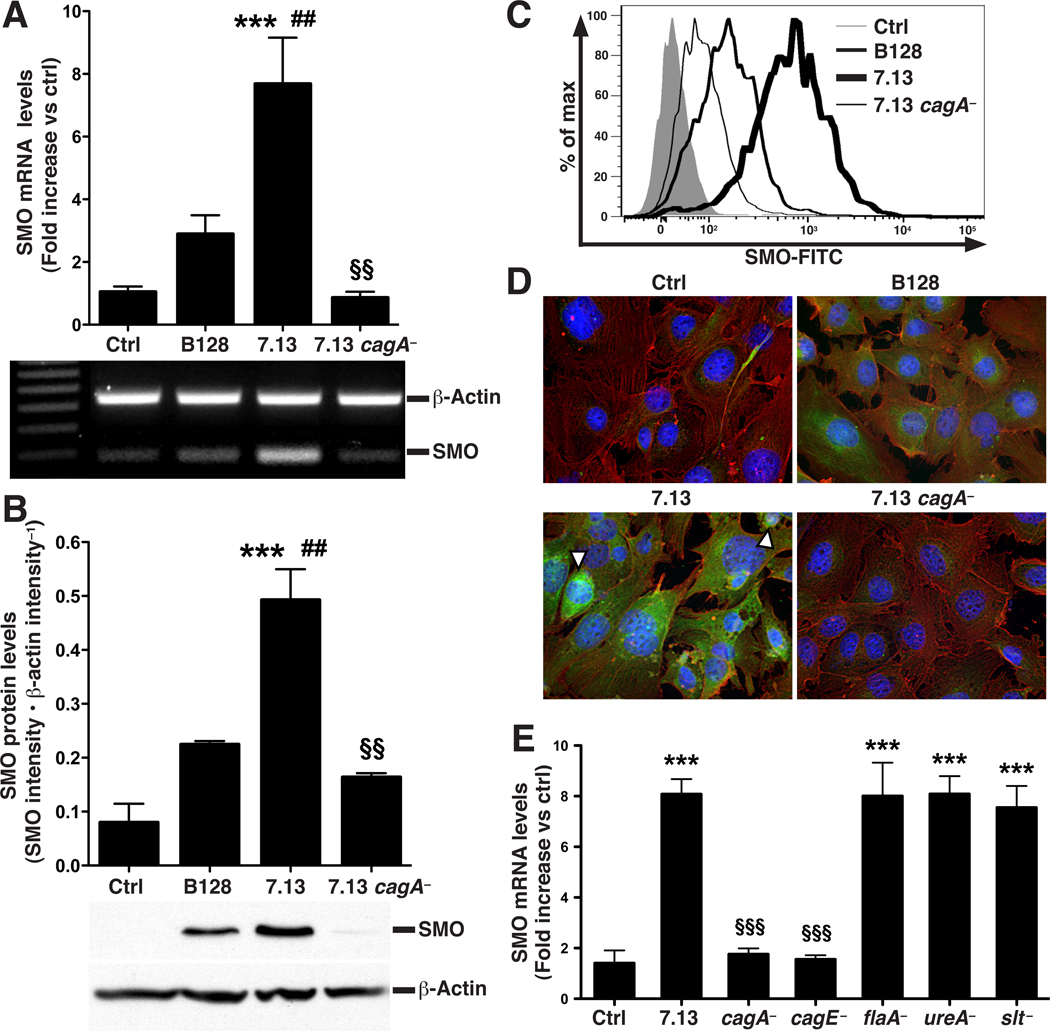
We have previously implicated SMO induction by H. pylori in the oxidative stress that occurs in infected gastric cancer epithelial cell lines.9 In order to address the role of CagA in SMO induction and its biological effects, we now used conditionally-immortalized gastric epithelial cells (ImSt cells) exposed to the carcinogenic H. pylori strain 7.13. When ImSt cells were co-cultured with 7.13, higher levels of CagA were detected, compared to when its non-carcinogenic parental strain B128 was tested (Supplementary Figure 1A).20 Co-culture of 7.13 with ImSt cells increased SMO mRNA expression by 8-fold, whereas B128 caused less than a 3-fold increase, and a cagA isogenic mutant of 7.13 (7.13 cagA−) did not induce SMO mRNA expression (Figure 1A). Similarly, when assessed by Western blotting, 7.13 significantly upregulated SMO protein levels, which were induced to a lesser degree with B128, and 7.13 cagA− did not increase SMO protein levels (Figure 1B). We confirmed these findings with flow cytometric analysis of SMO protein levels (Figure 1C) and with immunofluorescence staining for SMO (Figure 1D). ImSt cells co-cultured with 7.13 displayed intense cytoplasmic accumulation of SMO protein with perinuclear localization also apparent (Figure 1D); SMO staining was substantially less in cells cultured with B128 and absent when 7.13 cagA− was used. 7.13 cagE− also failed to induce SMO expression, whereas mutants of flaA, ureA, or slt, which inhibits peptidoglycan synthesis, induced SMO mRNA expression to the same level as wild-type 7.13 (Figure 1E).
Figure 1.
Induction of SMO in ImSt cells co-cultured with H. pylori strains at a MOI of 200. (A) Real-time PCR (upper panel), and semi-quantitative reverse transcription PCR (lower panel) for SMO mRNA expression assessed at 6 h. (B) Upper panel, densitometry; lower panel, representative Western blot for SMO and β-actin. (C) Flow cytometric analysis of SMO protein. (D) Immunofluorescent detection of SMO; cells were stained for SMO and β-actin, detected with secondary antibodies conjugated with FITC (green) and rhodamine (red), respectively. Nuclei were stained with DAPI (blue). Examples of perinuclear and nuclear staining for SMO are denoted by white arrows. (E) Cells were cultured with isogenic mutants of 7.13. SMO mRNAe xpression was measured by real-time PCR after 6 h. For A, B, and E, n = 3 in duplicate; ***P < .001 versus control (Ctrl); ##P < .01 versus B128; §§P < .01, §§§P < .001 versus 7.13.
In addition, 7.13 induced SMO mRNA expression in a manner that was dependent on the multiplicity of infection (MOI) in ImSt cells, and 7.13 cagA− failed to induce SMO expression at each MOI from 10–200 (Supplementary Figure 1B). To test strain and cell line effects, we also co-cultured human AGS cells with H. pylori 60190, a cagA+ laboratory-adapted clinical strain21, and found that induction of SMO mRNA expression was significantly attenuated with the 60190 cagA− isogenic mutant in these cells, similar to the results with strain 7.13 (Supplementary Figure 1C).
H. pylori CagA-Induced Apoptosis Is SMO-Dependent
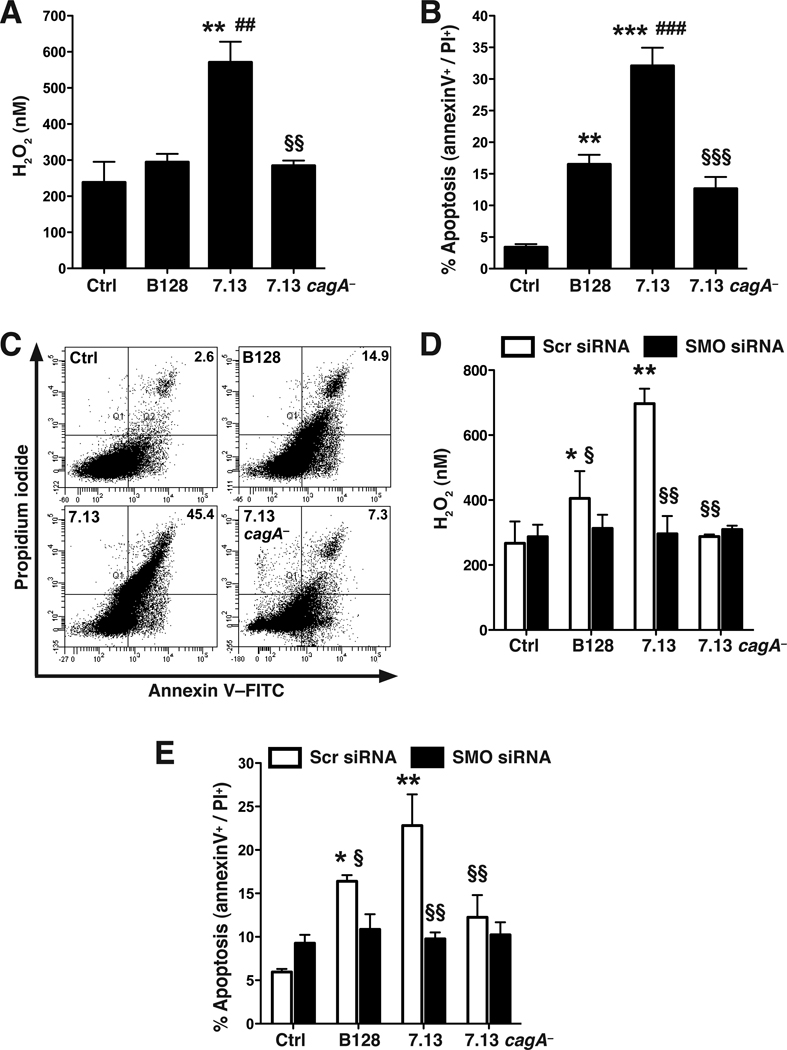
Our previous studies indicated that H. pylori-induced SMO expression and activity correlates with the time course of apoptosis in transformed AGS gastric epithelial cells.9 We assessed the role of SMO and cagA in ImSt cells, which exhibit a normal phenotype at 37°C.28 Co-culture of 7.13 with ImSt cells resulted in a significant increase in H2O2 production, whereas B128 and 7.13 cagA− did not cause this effect (Figure 2A). Consistent with our previous report that H2O2 derived from SMO results in apoptosis,8, 9 7.13 induced significantly more apoptosis than B128 or 7.13 cagA− (Figure 2B and C). The level of apoptosis induced by strain 7.13 increased in an MOI-dependent manner, and 7.13 cagA− induced significantly less apoptosis throughout the MOI range tested from 10–200 (Supplementary Figure 2A). When assessed in AGS cells, strain 60190-induced apoptosis was also significantly attenuated when its cagA− isogenic mutant was used (Supplementary Figure 2B). When ImSt cells were transfected with SMO siRNA (knockdown depicted in Supplementary Figure 3A and B), H2O2 and apoptosis were effectively reduced in cells stimulated with 7.13, while there was no reduction in cells stimulated with 7.13 cagA−, and only a small decrease in cells co-cultured with B128 (Figure 2D and E; Supplementary Figure 3C). Taken together, these data indicate that the CagA-dependent component of the apoptosis is mediated by SMO.
Figure 2.
Induction of H2O2 and apoptosis in ImSt cells by H. pylori and effect of SMO siRNA. Cells were co-cultured with H. pylori at an MOI of 200 for 24 h. (A) H2O2 in supernatants. (B) Summary data for apoptosis, and (C) Representative dot plots; percent of cells in the annexin V+/propidium iodide (PI)+ (late apoptosis) quadrants are indicated. (D) Levels of H2O2 in supernatants and (E) summary apoptosis data in cells transfected with scrambled (Scr) siRNA or SMO siRNA. In A, B, D, and E, n = 3 in duplicate. For A and B, **P < .01, ***P < .001 versus control; ##P < .01, ###P < .001 versus B128; and §§P < .01, §§§P < .001 versus 7.13. For D and E, *P < .05, **P < .01 versus uninfected cells transfected with scrambled siRNA; §P < .05, §§P < .01 versus cells transfected with scrambled siRNA and incubated with 7.13.
H. pylori-Induced DNA Damage Is Mediated by SMO and Is CagA-Dependent
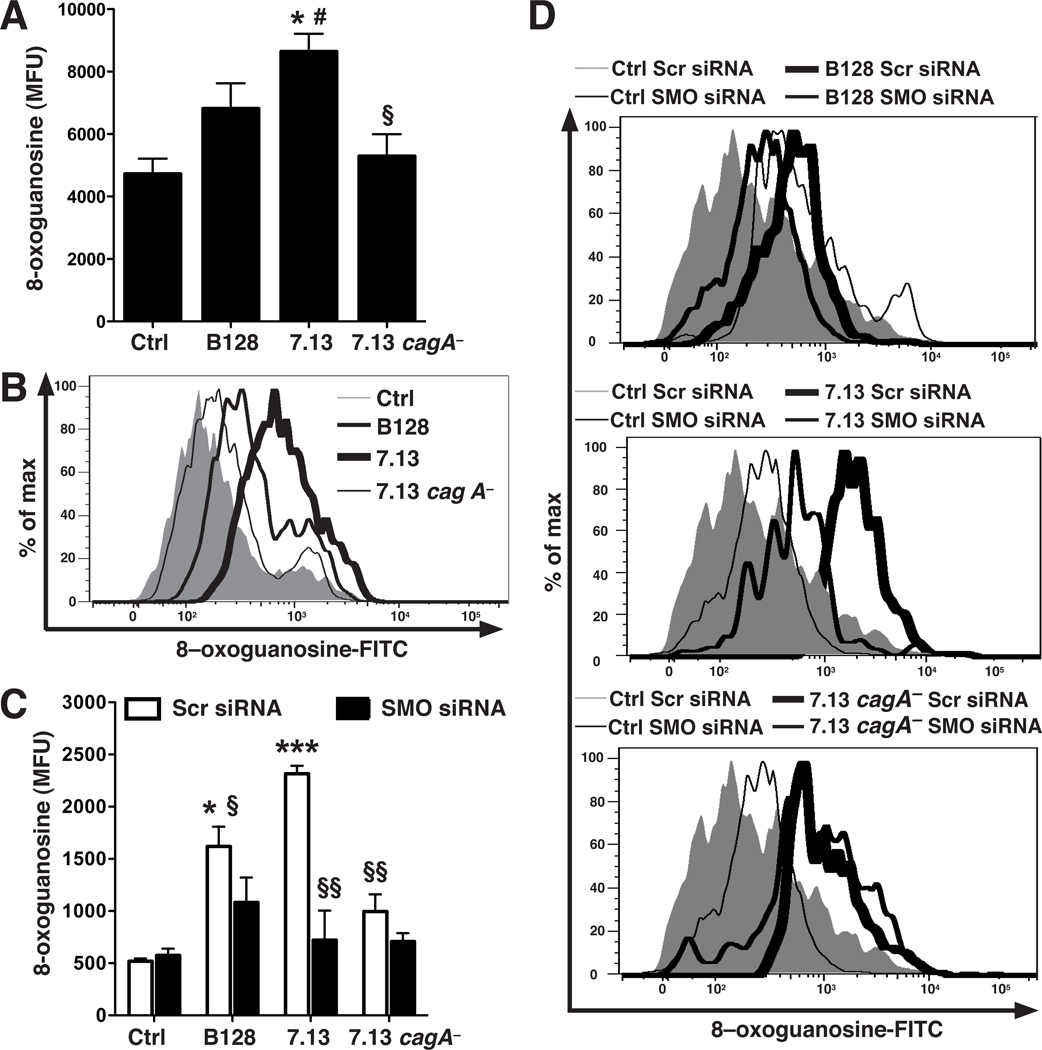
We utilized a specific assay for oxidative DNA damage, in which a FITC-labeled peptide that binds to 8-oxoguanosine is detected by flow cytometry.9 Strain 7.13 increased DNA damage, while B128 induced lower levels, and 7.13 cagA− did not cause DNA damage (Figure 3A and B). Strain 7.13 increased levels of DNA damage in a MOI-dependent manner that plateaued at MOI 200, and 7.13 cagA− failed to increase DNA damage at each MOI (Supplementary Figure 4A). When AGS cells were co-cultured with H. pylori 60190, there was also increased DNA damage, while 60190 cagA− had no effect (Supplementary Figure 4B). Knockdown of SMO by siRNA in ImSt cells resulted in a marked reduction in 7.13-induced DNA damage, whereas this knockdown resulted in only small decreases in DNA damage in cells co-cultured with the 7.13 cagA− or B128 strains (Figure 3C and D).
Figure 3.
Induction of oxidative DNA damage in ImSt cells by H. pylori and effect of SMO siRNA. Cells were co-cultured for 24 h with H. pylori at MOI 200. (A) Summary data for 8-oxoguanosine levels, in mean fluorescence units (MFU), measured by flow cytometry. (B) Representative histogram for 8-oxoguanosine levels. (C) Summary data for DNA damage in cells transfected with siRNA. (D) Representative histograms for 8-oxoguanosine levels in transfected cells. In A and C, n = 3 in duplicate. For A, *P < .05 versus control, #P < .05 versus B128, §P < .05 versus 7.13. For C, *P < .05, ***P < .001 versus uninfected ImSt cells transfected with scrambled siRNA; §P < .05, §§P < .01 versus cells transfected with scrambled siRNA and incubated with 7.13.
Ectopic Expression of CagA Increases SMO Expression, Apoptosis, and DNA Damage
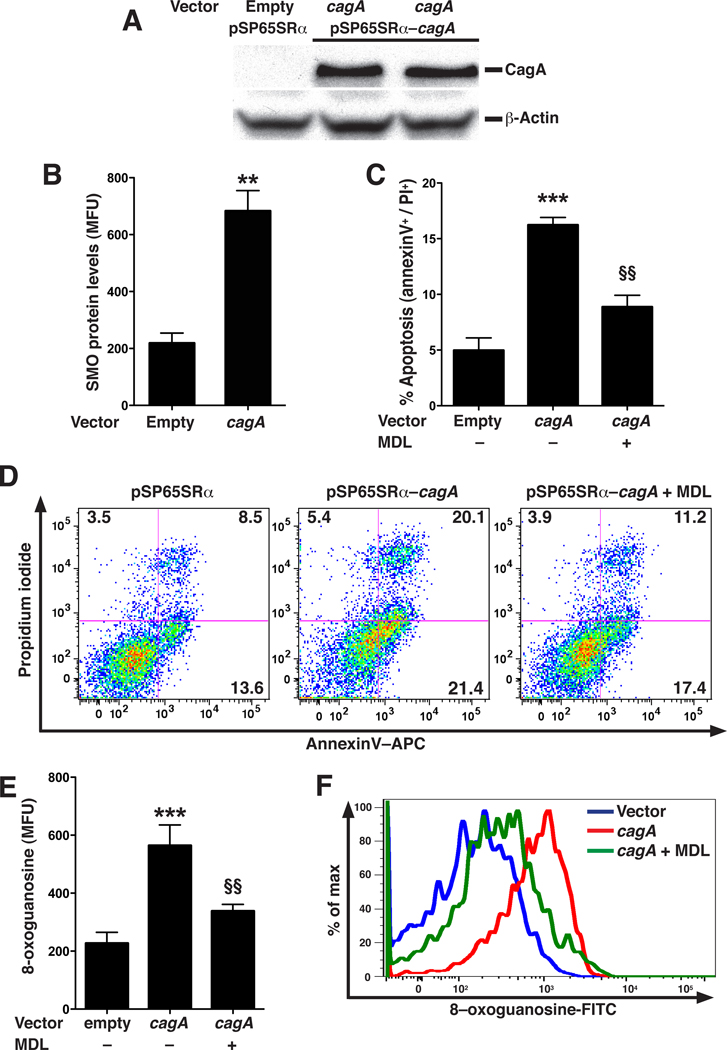
We directly tested the effects of CagA in ImSt cells, using co-transfection of cagA and EGFP expression vectors, and analyzing cells expressing EGFP, indicative of successful transfection, by flow cytometry. Exogenous expression of cagA increased levels of CagA protein (Figure 4A), SMO protein (Figure 4B) and apoptosis (Figure 4C and D) in parallel, when compared to empty vector alone. MDL 72527, an inhibitor of SMO,9 decreased CagA-induced apoptosis (Figure 4C and D). Similarly, expression of CagA increased DNA damage that was inhibited with MDL 72527 (Figure 4E and F). EGFP-negative cells did not show increases in levels of SMO protein, apoptosis, or DNA damage (Supplementary Figure 5A–C).
Figure 4.
Ectopic expression of cagA in ImSt cells and effect on apoptosis and DNA damage. (A) Representative Western blot for CagA and β-actin in ImSt cells transfected with empty vector and CagA expression vector. (B–F) Cells were co-transfected with EGFP and cagA vectors, and data were analyzed from EGFP+ cells 24 h later. (B) SMO expression levels analyzed by flow cytometry. (C) Summary apoptosis data in cells ± the SMO inhibitor MDL 72527 (250 µM). (D) Representative dot plots of annexin V versus PI for apoptosis measurement. (E) Summary data for oxidative DNA damage. (F) Representative histogram for 8-oxoguanosine levels. In B, C, and E, n = 2 in duplicate; **P < .01, ***P < .001 versus cells transfected with empty vector; in C and E, §§P < .01 versus cells transfected with cagA vector.
H. pylori Infection Increases SMO Expression, DNA Damage, and Apoptosis in Human Gastric Tissues in a CagA-Dependent Manner
To provide further biological relevance, we determined levels of SMO, DNA damage, and apoptosis in gastric biopsies from human subjects in which H. pylori infection status and the presence or absence of cagA in cultured isolates was assessed by PCR.20 Two of the eight strains that were PCR-positive did not express CagA protein (Supplementary Figure 6A); these strains were classified as cagA−. Using a TaqMan-based assay25 there was a substantial (two-log-order) increase in SMO mRNA expression levels in gastric biopsies from patients infected with cagA+ H. pylori compared to normal gastric tissues, and these levels were not upregulated in tissues from patients infected with cagA− strains (Figure 5A). When co-cultured with AGS cells, all clinical isolates expressing CagA translocated this protein, as indicated by detectable phosphorylated CagA in cell lysates (Supplementary Figure 6B). To assess functionality of CagA in vivo, IL-8 mRNA expression in the human gastric tissues was measured (Supplementary Figure 6C) and it paralleled the levels of CagA and phosphorylated CagA in AGS cells. There was increased SMO mRNA expression in biopsies from patients infected with cagA+ H. pylori strains (Figure 5A). SMO mRNA levels in gastric tissues were positively correlated with the amounts of CagA or phosphorylated CagA detected in AGS cell lysates co-cultured with these strains (Supplementary Figure 6D and E).
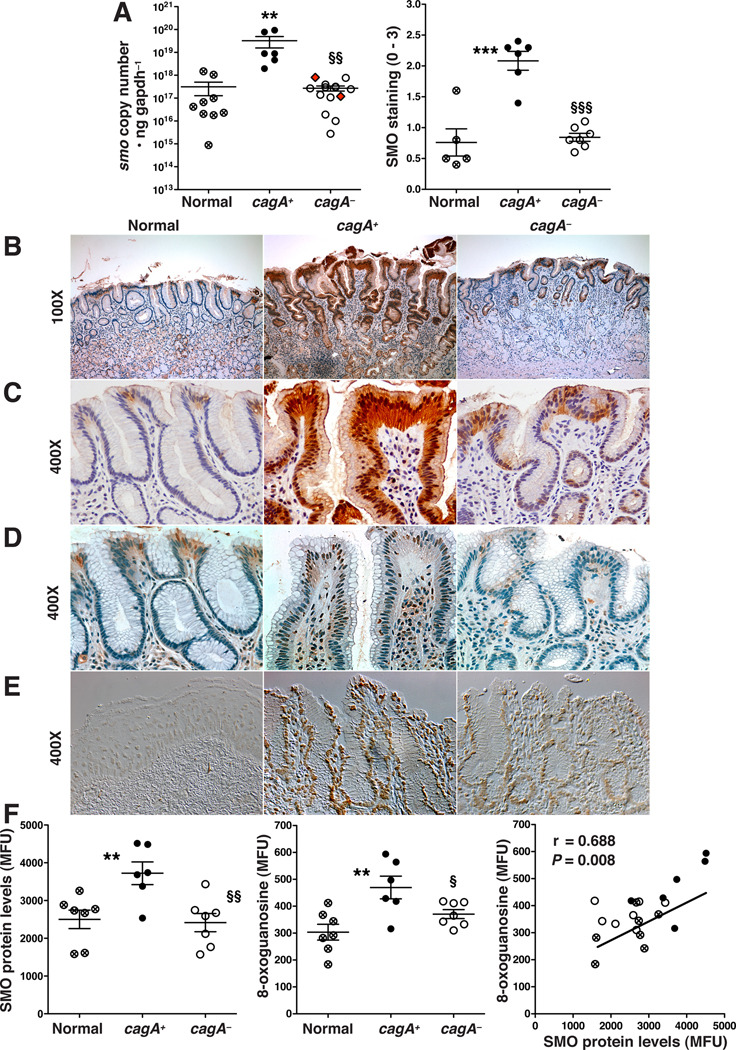
Figure 5.
Levels of SMO, DNA damage, and apoptosis in human antral gastric biopsies. (A) Left panel: SMO mRNA expression measured by TaqMan PCR; red diamonds indicate strains PCR-positive for cagA, but CagA protein-negative (Supplementary Figure 6A); right panel: scoring of SMO staining intensity in epithelial cells from immunohistochemistry analysis. (B, and C) Immunohistochemistry for SMO. (D) Immunohistochemistry for 8-OHdG. (E) Levels of apoptosis by ISOL, imaged by DIC microscopy. (F) Gastric epithelial cells were isolated from gastric tissues and analyzed by flow cytometry. Left panel: levels of SMO; middle panel: levels of DNA damage; right panel: correlation (Pearson coefficient) between SMO and DNA damage levels. In A and F, each circle is a different patient; **P < .01, ***P < .001 versus uninfected normal tissues, §P < .05, §§P < .01, §§§P < 0.001 versus cagA-positive tissues.
Immunostaining of gastric tissues indicated increased SMO protein levels in epithelial cells from patients infected with cagA+ H. pylori compared to normal subjects or those infected with cagA− strains (Figure 5A–C). As shown in Figure 5B, SMO staining in normal gastric tissues was weak and restricted to the surface epithelium, was strongly increased in surface epithelial cells and within deep glands in patients infected with cagA+ H. pylori, and was diminished in patients infected with cagA− H. pylori. Both cytoplasmic and nuclear localization of SMO was detected on high power images (Figure 5C). There was also strong staining for the DNA damage marker, 8-OHdG (Figure 5D; data quantified in Supplementary Figure 7), and for apoptosis, assessed by the ISOL technique (Figure 5E), in gastric biopsies from patients infected with cagA+ H. pylori that was markedly decreased in those infected with cagA− H. pylori or were uninfected.
SMO Expression and DNA Damage Correlate in Epithelial Cells Isolated from Human Gastric Biopsies
To further establish the relationship between SMO expression and DNA damage, we isolated epithelial cells from human gastric biopsies. When examined by flow cytometry, cells from patients infected with cagA+ H. pylori strains exhibited increased SMO protein expression and oxidative DNA damage (8-oxoguanosine levels) compared to cells isolated from normal subjects or patients harboring cagA− strains (Figure 5F). A plot of SMO versus 8-oxoguanosine levels demonstrated a significant positive correlation (Figure 5F).
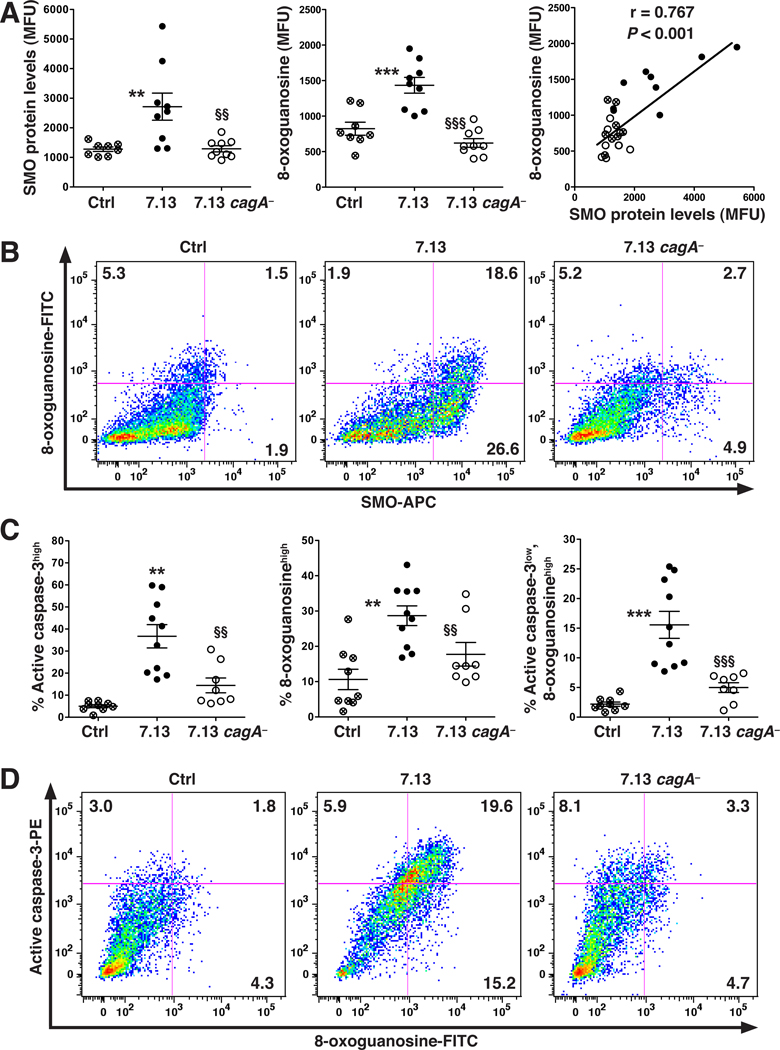
DNA Damage in Gerbils Is CagA-dependent and Is Present in a Subpopulation of Cells That Are Resistant to Apoptosis
Gerbils infected with H. pylori 7.13 developed gastric dysplasia or carcinoma (Supplementary Figure 8). When gastric epithelial cells isolated from these animals were analyzed by flow cytometry, there were increased SMO protein and 8-oxoguanosine levels that were strongly correlated, while cells from animals infected with the 7.13 cagA− strain and possessing only gastritis exhibited no such increases (Figure 6A, 6B; Supplementary Figure 8). As shown in Figure 6B, the majority of the 8-oxoguanosinehigh cells from 7.13-infected gerbils were SMOhigh (93.8 ± 0.9%; representative plots in Supplementary Figure 9). Neoplastic transformation of cells requires DNA alterations as well as circumvention of apoptosis. To monitor such changes we simultaneously analyzed active caspase-3, a marker of apoptosis, and DNA damage in the same cells. Wild-type 7.13, but not 7.13 cagA−, caused an increase in both markers, including a subset of cells that were 8-oxoguanosinehigh, active caspase-3high (Figure 6C and 6D). Importantly, a substantial percentage of the 8-oxoguanosinehigh cells were also active caspase-3low (44.2 ± 3.1%; representative plots in Supplementary Figure 10), identifying a subpopulation of cells with DNA damage that were apoptosis-resistant.
Figure 6.
Levels of SMO protein, DNA damage, and apoptosis in gerbils infected with 7.13 or 7.13 cagA− at 14 weeks post-inoculation. In the 7.13 group, 5 gerbils had invasive carcinoma and 5 had dysplasia, while in the 7.13 cagA− group all had chronic gastritis only. (A) Left and middle panels: levels of SMO protein and 8-oxoguanosine, respectively, measured by flow cytometry; right panel: correlation (Pearson coefficient) between SMO and 8-oxoguanosine levels. (B) Representative dot plots. (C) Percentage of cells active caspase-3high (left panel); 8-oxoguanosinehigh (middle panel); active caspase-3low,8-oxoguanosinehigh (right panel). (D) Representative dot plots. In A and C, each circle is a different gerbil; **P < .01, ***P < .001 versus uninfected control; §§P < .01, §§§P < .001 versus gerbils infected with 7.13.
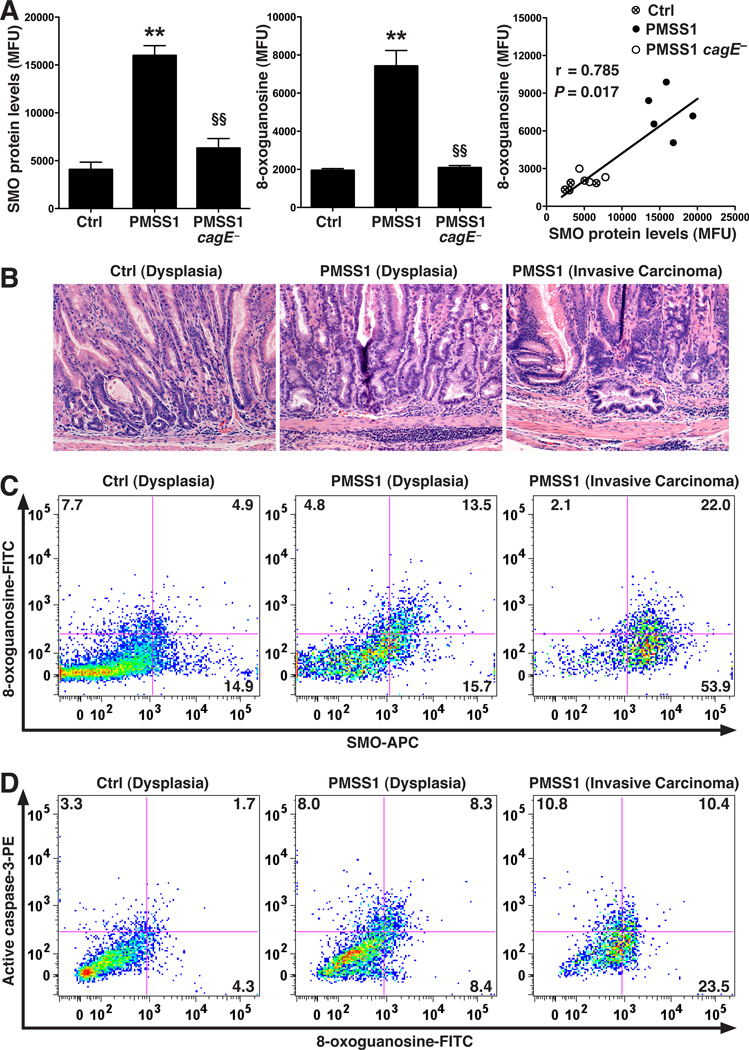
H. pylori Induces SMO in Mice in a CagA-dependent Manner and Also Leads to Generation of Cells With DNA Damage and Resistance to Apoptosis
H. pylori strain PMSS1 can translocate CagA into gastric epithelial cells after in vivo passage and is an excellent colonizer of mice.22 We examined gastric epithelial cells isolated from mice 2 months after infection of 6–8 week old mice with PMSS1 or its cagE isogenic mutant that fails to translocate CagA,22 and found that PMSS1 infection led to increased levels of SMO protein, active caspase-3, and 8-oxoguanosine, whereas cells from mice infected with PMSS1 cagE− exhibited no significant increase in these parameters (Figure 7A; Supplementary Figure 11A–C). There were no differences in colonization levels between wild-type and cagE− PMSS1 (data not shown). There was a significant positive correlation between SMO and 8-oxoguanosine levels (Figure 7A) and the increase in the latter with PMSS1 infection was detected in cells expressing high levels of SMO and was absent in mice infected with PMSS1 cagE− (Supplementary Figure 11B).
Figure 7.
SMO and DNA damage levels in gastric epithelial cells in mice. (A) C57BL/6 mice were infected with PMSS1 or its cagE isogenic mutant, and gastric epithelial cells were isolated at 2 months post-inoculation. Left and middle panels: summary data for levels of SMO protein and 8-oxoguanosine, respectively, measured by flow cytometry. Right panel: correlation (Spearman coefficient) between levels of SMO protein and 8-oxogaunosine. (B–D) FVB/N INS-GAS mice infected with PMSS1 for 4 months. (B) H&E staining showing dysplastic and dilated glands in infected and uninfected mice, and invasive carcinoma in H. pylori-infected mice. (C) Representative dot plots of SMO and 8-oxoguanosine levels in gastric epithelial cells. (D) Representative dot plots of active caspase-3 and 8-oxoguanosine levels. In A, n = 5 per group; In B–D, n = 3 uninfected and 6 infected mice. For A, **P < .01 versus control, §§P < .01 versus PMSS1.
We used hypergastrinemic INS-GAS mice to assess levels of SMO and DNA damage in gastric epithelial cells because these mice develop neoplasia (Figure 7B). In the uninfected mice with dysplasia, the percentage of cells that were SMOhigh,8-oxoguanosinehigh was low compared to the percentage in the H. pylori PMSS1-infected INS-GAS mice (Figure 7C). Most of the increase in the percentage of cells with DNA damage with infection derived from the SMOhigh population (Figure 7C; Supplementary Figure 12). The SMOhigh, 8-oxoguanosinehigh cell population was further increased with carcinoma (Figure 7C). A substantial population of 8-oxoguanosinehigh, active-caspase-3low cells was present in PMSS1-infected INS-GAS mice with carcinoma; this population was more modest in infected mice with dysplasia and nearly absent (2.6 ± 1.7% of cells) in uninfected mice (Figure 7D).
Additionally, incubation of ImSt cells with 7.13 increased the population of 8-oxoguanosinehigh, active-caspase-3low cells (19.8 ± 2.3% of cells) in vitro, while 7.13 cagA− did not increase these cells above basal levels (Supplementary Figure 13A). The 8-oxoguanosinehigh, active-caspase-3low cells expressed higher levels of the anti-apoptotic protein Bcl-2 than 8-oxoguanosinelow, active-caspase-3low cells (Supplementary Figure 13B and 13C). Gating on active caspase-3low cells (Supplementary Figure 14A) demonstrated that with 7.13 stimulation there was a significant increase in the Bcl-2high population, which was not observed in cells cultured with 7.13 cagA− (Supplementary Figure 14B–C). Further, 8-oxoguanosine levels were increased in the active caspase-3low, Bcl-2high versus active caspase-3low, Bcl-2low cells (Supplementary Figure 14D).
Discussion
Our current findings implicate SMO as a causal link between CagA and gastric carcinogenesis. We have shown that in non-transformed gastric epithelial cells, H. pylori strain 7.13 induces more SMO expression and associated H2O2 production, apoptosis, and DNA damage than the parental strain B128, which is a poor translocator of CagA. The essential role of CagA was supported by our findings that cagA isogenic mutants of strains 7.13 and 60190 exhibited profound attenuation of SMO inducing ability. The role of SMO as a causal factor in generation of oxidative stress and its consequences was demonstrated by the potent effects of SMO knockdown in abrogating CagA-dependent H2O2 production, apoptosis, and DNA damage. The link between CagA, SMO, and its biologic effects was further evidenced by our findings that ectopic expression of cagA directly induced SMO, and that the associated apoptosis and DNA damage was blocked by an SMO inhibitor.
While flaA, ureA, and slt isogenic mutants exhibited no attenuation of SMO induction, the 7.13 cagE− mutant resulted in reduced SMO levels similar to those occurring with the cagA− mutant. CagE is an essential component of the H. pylori type IV secretion system and is required for translocation of CagA.22 cagA+ H. pylori strains are associated with increased production of reactive oxygen species (ROS) in human gastric mucosa,26 though no mechanism linking the induction of ROS and cagA+ infection has been elucidated. While previous studies have demonstrated increased ROS in transformed gastric epithelial cell lines,9, 10 we now report that H2O2, specifically, is increased in non-malignant ImSt cells and that this is a CagA-dependent process. Furthermore, in addition to cytoplasmic staining, SMO also localized to the perinuclear region and the nucleus of 7.13-infected ImSt cells; this finding is consistent with the report of a nuclear isoform of SMO,25, 27 and indicates that such proximity to cellular chromatin could increase the likelihood of DNA damage resulting from the release of H2O2 during spermine oxidation.
H. pylori infection has been reported to result in either increased or decreased levels of apoptosis.28,29 Our results demonstrate that H. pylori-induced apoptosis in ImSt cells is largely CagA-dependent, since 7.13 induced high levels of apoptosis, while B128 and 7.13 cagA− caused significantly less. However, levels were still higher with these two strains than in uninfected cells, suggesting that some apoptotic cell death is due to other bacterial factors that have been implicated as inducers in gastric epithelial cells.30 Our results implicating CagA as an important inducer of apoptosis differ from a recent report that exogenous overexpression of CagA can have a protective effect on apoptosis of gastric epithelial cells induced by etoposide or VacA.31 One factor is that the ImSt cells we used readily exhibit apoptosis, whereas transformed cell lines such as AGS cells are relatively apoptosis-resistant. MOI may also be a factor; we reported that a low MOI of H. pylori protects B lymphocytes from spontaneous apoptosis, while a higher MOI has the opposite effect.32 However, in the current study we found a reduction in both apoptosis and DNA damage with cagA− strains across a broad range of MOIs. It has been reported that ectopic expression of CagA causes apoptosis in gastric epithelial cells;33 we now show that this occurs in a SMO-dependent manner.
In addition to dysregulation of apoptosis, another essential step in neoplastic transformation is accumulation of genetic alterations that can result from oxidative DNA damage. Studies in Big Blue transgenic mice demonstrated that H. pylori infection causes base substitution and frame-shift mutations.12 While cagA+ infection has been linked to DNA damage in humans,34 the responsible biochemical and cellular mechanisms have not been identified. We have directly implicated SMO in this process in epithelial cells. This was shown by siRNA knockdown and inhibitor studies in vitro, and our findings that exogenous overexpression of CagA caused epithelial cell DNA damage that could be prevented with an SMO inhibitor.
We demonstrated the in vivo relevance of our findings as we observed increased SMO mRNA expression and intense nuclear and perinuclear SMO staining in biopsies from humans infected with cagA+, but not cagA− strains. By both immunostaining and flow cytometry, we demonstrated more oxidative DNA damage in gastric epithelial cells with high levels of SMO expression; the strong correlation between these two events indicates that they are linked. Neutrophils and macrophages produce ROS during the H. pylori immune response8 that may also damage DNA of adjacent gastric epithelial cells. However, ROS produced within gastric epithelial cells by SMO would be expected to be more damaging because of their close proximity to the DNA, and they may thus be an important factor in tumorigenesis. Also, the increase in epithelial SMO we show in vivo may be a specific response to H. pylori, as we have reported that in colon tissues from patients with ulcerative colitis, SMO was primarily upregulated in mononuclear cells, but not epithelial cells.25 We also demonstrated a strong link between CagA, SMO, and DNA damage in two experimental models of H. pylori infection, namely mice infected with H. pylori PMSS1, which translocates CagA,22 and gerbils infected with 7.13. In both models the wild-type strains resulted in a strong correlation between SMO and 8-oxoguanosine levels, and mice infected with PMSS1 cagE−, a strain incapable of CagA translocation,22 and gerbils infected with 7.13 cagA− exhibited markedly lower levels of SMO and oxidative DNA damage, providing further evidence that CagA plays a major role in the induction of SMO-driven DNA damage.
Dysregulation of apoptosis has the potential to be deleterious, since increased apoptosis would be expected to increase the likelihood of development of gastric atrophy and potentially, increased epithelial cell turnover, while protection from apoptosis could increase risk for neoplastic transformation.4 Varying results have been reported related to the effect of CagA on apoptosis in the human stomach28,29, some of which found no association between CagA and increased apoptosis during infection. In the current report, we found a modest increase in apoptosis in our human samples with gastritis. Such differing results may be due to differences in the methodology used to detect apoptosis, location of biopsies, and histopathologic stage of disease. In the gerbil model of gastric dysplasia and carcinoma, we discovered that while infection with the cagA+ strain 7.13 also resulted in more epithelial apoptosis than cagA− infection, there was a subpopulation of cells that exhibited high levels of DNA damage and low levels of apoptosis (8-oxoguanosinehigh, active-caspase-3low) that was only present in cells from gerbils infected with the cagA+ strain. Moreover, we also observed this subpopulation of cells in INS-GAS mice infected with PMSS1, another strain capable of translocating CagA into gastric epithelial cells, as well as in ImSt cells infected with 7.13, but not with 7.13 cagA−. Taken together, these findings indicate that while H. pylori causes apoptosis in gastric epithelial cells, there is also a substantial fraction of cells infected with cagA+ strains that are protected from apoptosis during infection.
These differences in susceptibility to apoptosis may derive in part from the heterogeneity of the gastric epithelial cell populations in the stomach,35 but another important factor may be alterations in pro- and anti-apoptotic signaling pathways.4 In the present study, we found that 8-oxoguanosinehigh, active caspase-3low populations of ImSt cells activated with 7.13 had increased expression of anti-apoptotic Bcl-2. Our group has reported that epidermal growth factor receptor activation during H. pylori infection protects gastric epithelial cells from apoptosis.7 Moreover, we have found that inhibition of EGFR transactivation attenuates both SMO expression and the 8-oxoguanosinehigh, active caspase-3low cell population that we have described herein (data not shown).
Gastric carcinogenesis is a multifactorial disease and other contributors to the pathologic process could also be involved, including include epigenetic changes occurring within the epithelium,36 changes in specific genes in the gastric microenvironment,37 or expansion of stomach pluripotent stem cells38 or bone marrow-derived stem cells.39 Thus, a compensatory increase in proliferation of stem cells in the stomach due to increased epithelial cell apoptosis during H. pylori infection may contribute to neoplastic potential. The current study provides insights into the mechanisms of interactions between H. pylori CagA and the biochemical pathways of DNA damage in epithelial cells. These findings may be useful in identifying new therapies to prevent gastric carcinogenesis, including interference with polyamine synthesis40 to prevent generation of SMO substrate, or direct inhibition of SMO.
Supplementary Material
Acknowledgments
Funding: Supported by National Institutes of Health grants R01DK053620 and R01AT004821 (to K.T.W.), P01CA028842 (to K.T.W and P.C.), P01CA116087 (to R.M.P., D.P.B., K.T.W.), R01DK056008 and R01DK066176 (to D.B.P.), R01CA077955 and R01DK058587 (to R.M.P.) R01CA051085 and R01CA098454 (to R.A.C.), the Flow Cytometry Core of the Vanderbilt University Digestive Disease Research Center grant (P30DK058404), UL1RR024975 (Vanderbilt CTSA, Pilot Project to KTW), a Merit Review Grant from the Office of Medical Research, Department of Veterans Affairs (to K.T.W.), and the Philippe Foundation (T.S. and A.P.G.).
Abbreviations
- SMO
spermine oxidase
- CagA
cytotoxin-associated gene A
- PMSS1
pre-mouse Sydney strain 1
- INS-GAS
Insulin-Gastrin
- EGFP
enhanced green fluorescence protein
- ISOL
in situ oligo ligation
- PI
propidium iodide
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- ImSt
immortalized stomach
- FITC
fluorescein isothiocyanate
- MFU
mean fluorescence units
- MOI
multiplicity of infection
- PCR
polymerase chain reaction
- Scr
scrambled
- siRNA
small interfering RNA
- ROS
reactive oxygen species
Footnotes
Conflicts of Interest: None
Writing Assistance: None
Author contributions:
Rupesh Chaturvedi: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis
Mohammad Asim: acquisition of data; technical support
Judith Romero-Gallo: material support, acquisition of data
Daniel P. Barry: critical revision of manuscript for intellectual content
Svea Hoge: acquisition of data
Thibaut de Sablet: acquisition of data
Alberto G. Delgado: acquisition of data
Lydia E. Wroblewski: acquisition of data
M. Blanca Piazuelo: acquisition of data
Fang Yan: material support, acquisition of data
Dawn A. Israel: material support, critical revision of manuscript for intellectual content
Robert A. Casero, Jr.: material support, and critical revision of manuscript for intellectual content
Pelayo Correa: material support, obtained funding
Alain P. Gobert: acquisition of data, critical revision of manuscript for intellectual content
D. Brent Polk: material support, critical revision of manuscript for intellectual content, obtained funding
Richard M. Peek, Jr.: material support, study concept and design, critical revision of manuscript for intellectual content, obtained funding
Keith T. Wilson: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of manuscript for important intellectual content, statistical analysis, obtained funding, study supervision
References
- 1.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 3.Peek RM, Jr, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss SF, Calam J, Agarwal B, et al. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner S, Beil W, Westermann J, et al. Regulation of gastric epithelial cell growth by Helicobacter pylori : offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 7.Yan F, Cao H, Chaturvedi R, et al. Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori -induced apoptosis. Gastroenterology. 2009;136:1297–1307. doi: 10.1053/j.gastro.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi R, Cheng Y, Asim M, et al. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Chaturvedi R, Cheng Y, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 10.Bagchi D, Bhattacharya G, Stohs SJ. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic Res. 1996;24:439–450. doi: 10.3109/10715769609088043. [DOI] [PubMed] [Google Scholar]
- 11.Farinati F, Cardin R, Degan P, et al. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998;42:351–356. doi: 10.1136/gut.42.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touati E, Michel V, Thiberge JM, et al. Chronic Helicobacter pylori infections induce gastric mutations in mice. Gastroenterology. 2003;124:1408–1419. doi: 10.1016/s0016-5085(03)00266-x. [DOI] [PubMed] [Google Scholar]
- 13.Wei J, Nagy TA, Vilgelm A, et al. Regulation of p53 tumor suppressor by Helicobacter pylori in gastric epithelial cells. Gastroenterology. 2010;139:1333–1343. doi: 10.1053/j.gastro.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amieva MR, Vogelmann R, Covacci A, et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odenbreit S, Puls J, Sedlmaier B, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 16.Kuipers EJ, Perez-Perez GI, Meuwissen SG, et al. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 17.Parsonnet J, Friedman GD, Orentreich N, et al. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 19.Ohnishi N, Yuasa H, Tanaka S, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco AT, Israel DA, Washington MK, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peek RM, Jr, Blaser MJ, Mays DJ, et al. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999;59:6124–6131. [PubMed] [Google Scholar]
- 22.Arnold I, Lee JY, Amieva MR, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140:199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitehead RH, VanEeden PE, Noble MD, et al. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci U S A. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong SK, Chaturvedi R, Piazuelo MB, et al. Increased expression and cellular localization of spermine oxidase in ulcerative colitis and relationship to disease activity. Inflamm Bowel Dis. 2010;16:1557–1566. doi: 10.1002/ibd.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies GR, Simmonds NJ, Stevens TR, et al. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray-Stewart T, Wang Y, Goodwin A, et al. Nuclear localization of human spermine oxidase isoforms - possible implications in drug response and disease etiology. Febs J. 2008;275:2795–2806. doi: 10.1111/j.1742-4658.2008.06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirin H, Sordillo EM, Oh SH, et al. Helicobacter pylori inhibits the G1 to S transition in AGS gastric epithelial cells. Cancer Res. 1999;59:2277–2281. [PubMed] [Google Scholar]
- 29.Peek RM, Jr, Moss SF, Tham KT, et al. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 30.Cover TL, Krishna US, Israel DA, et al. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951–957. [PubMed] [Google Scholar]
- 31.Oldani A, Cormont M, Hofman V, et al. Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells. PLoS Pathog. 2009;5:e1000603. doi: 10.1371/journal.ppat.1000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bussiere FI, Chaturvedi R, Asim M, et al. Low multiplicity of infection of Helicobacter pylori suppresses apoptosis of B lymphocytes. Cancer Res. 2006;66:6834–6842. doi: 10.1158/0008-5472.CAN-05-4197. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsumi R, Higashi H, Higuchi M, et al. Attenuation of Helicobacter pylori CagA•SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J Biol Chem. 2003;278:3664–3670. doi: 10.1074/jbc.M208155200. [DOI] [PubMed] [Google Scholar]
- 34.Ladeira MS, Rodrigues MA, Salvadori DM, et al. Relationships between cagA, vacA, and iceA genotypes of Helicobacter pylori and DNA damage in the gastric mucosa. Environ Mol Mutagen. 2004;44:91–98. doi: 10.1002/em.20045. [DOI] [PubMed] [Google Scholar]
- 35.Mimuro H, Suzuki T, Nagai S, et al. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe. 2007;2:250–263. doi: 10.1016/j.chom.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 37.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiao XT, Ziel JW, McKimpson W, et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 40.Chaturvedi R, Asim M, Hoge S, et al. Polyamines impair immunity to Helicobacter pylori by inhibiting L-arginine uptake required for nitric oxide production. Gastroenterology. 2010;139:1686–1698. doi: 10.1053/j.gastro.2010.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.