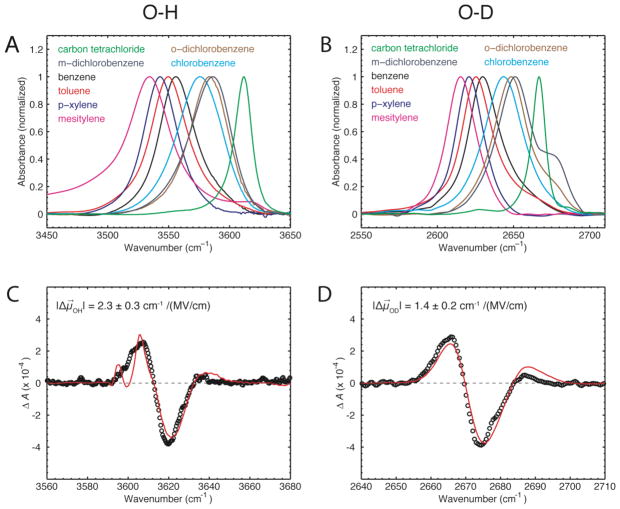
Figure 1.
(A, B) Experimental FTIR spectra of 200 mM phenol dissolved in different organic solvents. The OH/OD stretch mode of free phenol in carbon tetrachloride is shown in green. The OH/OD stretch modes for phenol in aromatic solvents are all red-shifted. The shoulder of phenol-OD in m- and o-dichlorobenzene might arise either from a Fermi resonance or a second geometry of the complex in these solvents. (C, D) Vibrational Stark spectra of 2,6-di-t-butylphenol (approximately 1:1 OH and OD) in toluene measured at an applied field of 1 MV/cm at T = 77 K (black) with fit (red).