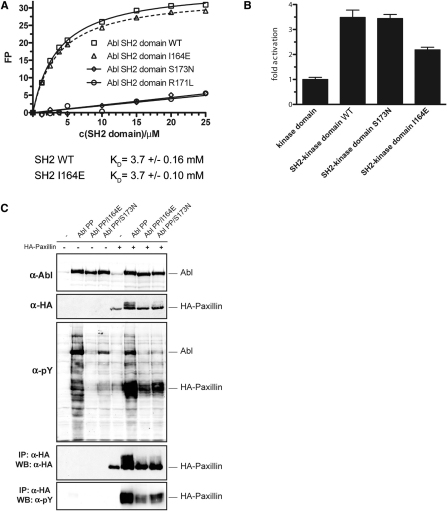
Figure S2.
The I164E Mutation Does Not Interfere with the Phosphotyrosine-Binding Capability of the Abl SH2 Domain, Related to Figure 2
(A) Fluorescence polarization binding assay of recombinant WT and mutant Abl SH2 domains to a fluorescently labeled tyrosine phosphorylated peptide (EPPVpYANLS). The recombinant Abl SH2 domain harboring the I164E mutation was found to bind a phosphorylated peptide with equal affinity as the wt Abl SH2 domain. In contrast, Abl SH2 domains bearing the FLVRES mutants R171L or S173N were unable to bind tyrosine phosphorylated peptides.
(B) HEK293 cells were transfected with expression constructs comprising the Abl kinase domain alone or the SH2-kinase domain module of c-Abl (Abl SH2-kinase domain WT, S173N, I164E). Constructs were immunoprecipitated and subjected to in vitro kinase assays in the presence of an optimal Abl substrate peptide containing a single tyrosine phosphorylation site. In line with previous results, the SH2 domain had a positive role on the kinase activity, as we observed higher kinase activity of the Abl SH2-kinase domain construct as compared to the Abl kinase domain alone, and introduction of the I164E reverted this effect. However, the phosphotyrosine-binding mutant S173N did not show significantly different kinase activity as compared to the WT Abl SH2-kinase domain construct. As the kinase substrate peptide used in this assay contains only one tyrosine residue, no (processive phosphorylation) effects dependent on the phosphotyrosine-binding capability of the Abl SH2 domain are observed. Together, these data rule out that the I164E mutation in the Abl SH2 domain leads to reduced kinase activity by interfering with phosphotyrosine binding of the SH2 domain. Furthermore, this experiment shows that the ability of the Abl SH2 domain to bind phosphotyrosine does not influence the in vitro kinase activity of the Abl kinase. Error bars represent SD.
(C) HEK293 cells were transiently transfected with the indicated c-Abl mutants in the presence or absence of a HA-tagged form of the Abl substrate paxillin. Total cell lysates were subjected to immunoblot analysis using antibodies against Abl, HA and phosphotyrosine. HA-tagged paxillin was immunoprecipitated from the same lysates and subjected to immunoblotting using antibodies against phosphotyrosine and HA. The Abl substrate paxillin contains multiple tyrosine residues that can serve as substrates for Abl. Thus, basal phosphorylation of one tyrosine residue might exert a positive feedback to Abl activity that is mediated by the Abl SH2 domain by binding the first phosphotyrosine and thereby positioning another unphosphorylated tyrosine close to the active site of the kinase. This phenomenon is referred to as processive phosphorylation and is dependent on the capability of the SH2 domain to bind to tyrosine-phosphorylated substrates. Paxillin was efficiently tyrosine phosphorylated by the constitutively active form of Abl (Abl PP) in a processive way (phosphorylation on multiple tyrosine residues as indicated by the shift of the HA-reactive bands to higher molecular weight species). Both Abl PP I164E and Abl PP S173N were able to phosphorylate paxillin at basal levels but neither of the mutants induced efficient processive phosphorylation of this substrate. This phenotype can be explained by the inability of the S173N mutant to bind to tyrosine-phosphorylated proteins, while in the case of the I164E mutant, this could be attributed to the lower overall activity of the kinase caused by the loss of the positive allosteric effect of the SH2 domain, which is independent of phosphotyrosine binding. In addition, this may indicate that for the recognition of Abl substrates with multiple phosphorylation sites the correct positioning of the SH2 domain appears to be of equal importance.