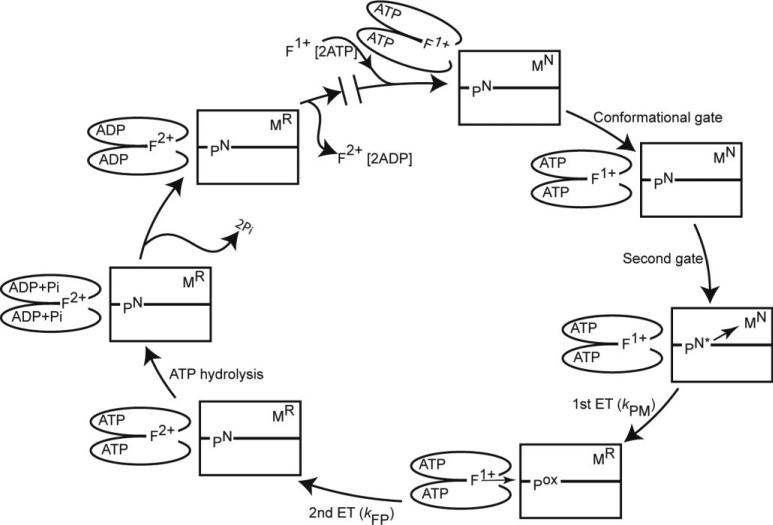
Figure 8. Models for nitrogenase mechanism.
Shown is one catalytic cycle for a functional half of nitrogenase composed of an αß-unit of the MoFe protein (rectangles) and a homodimeric Fe protein (ovals) with the associated metal clusters: F = [4Fe-4S] in the 1+ or 2+ oxidation states, P = P cluster in the N or ox oxidation states, and M = FeMo-cofactor in the N or R oxidation states. The cycle starts at the top with reduced Fe protein +2 MgATP associating with the αß-unit of the MoFe protein (1). The remaining steps are numbered (clockwise): (2) the conformational gate, (3) the second gate, (4) the first ET (kPM), (5) the backfill ET (kFP), (6) ATP hydrolysis, (7) release of 2 Pi, (1) and replacement of oxidized Fe protein + 2MgADP with reduced Fe protein + 2MgATP.